
 Copy to clipboard
Copy to clipboard 
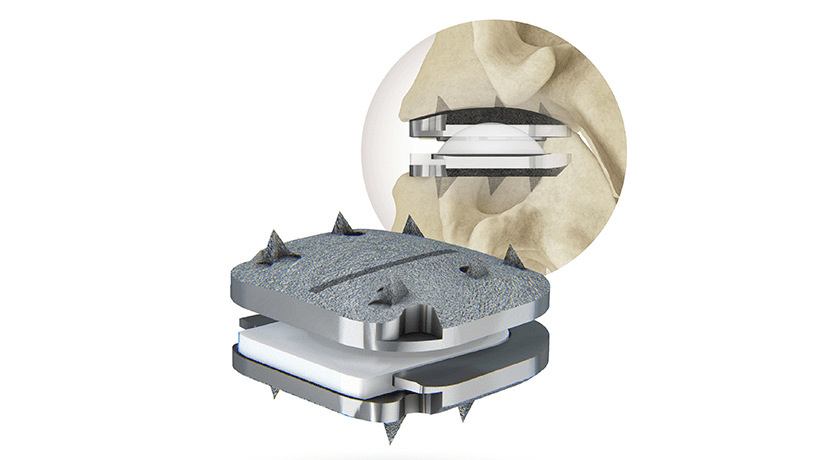
Centinel Spine announced the first implantation of its prodisc® C Vivo Cervical Total Disc Replacement (TDR) device. In July, the company received FDA approval for 1-level indications for prodisc C Vivo, prodisc C SK and prodisc C Nova.
The prodisc C Vivo has been in clinical use internationally since 2009 and is reported to be one of the most frequently implanted TDR devices outside of the U.S. The device has keel-less fixation and combines an anatomically-designed superior endplate with lateral spikes to optimize fit and provide immediate fixation.
Similar to all prodisc products, the prodisc C Vivo device incorporates prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 225,000 implantations worldwide.
Source: Centinel Spine, LLC
Centinel Spine announced the first implantation of its prodisc® C Vivo Cervical Total Disc Replacement (TDR) device. In July, the company received FDA approval for 1-level indications for prodisc C Vivo, prodisc C SK and prodisc C Nova.
The prodisc C Vivo has been in clinical use internationally since 2009 and is reported to be one of the...
Centinel Spine announced the first implantation of its prodisc® C Vivo Cervical Total Disc Replacement (TDR) device. In July, the company received FDA approval for 1-level indications for prodisc C Vivo, prodisc C SK and prodisc C Nova.
The prodisc C Vivo has been in clinical use internationally since 2009 and is reported to be one of the most frequently implanted TDR devices outside of the U.S. The device has keel-less fixation and combines an anatomically-designed superior endplate with lateral spikes to optimize fit and provide immediate fixation.
Similar to all prodisc products, the prodisc C Vivo device incorporates prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 225,000 implantations worldwide.
Source: Centinel Spine, LLC

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







