
 Copy to clipboard
Copy to clipboard 
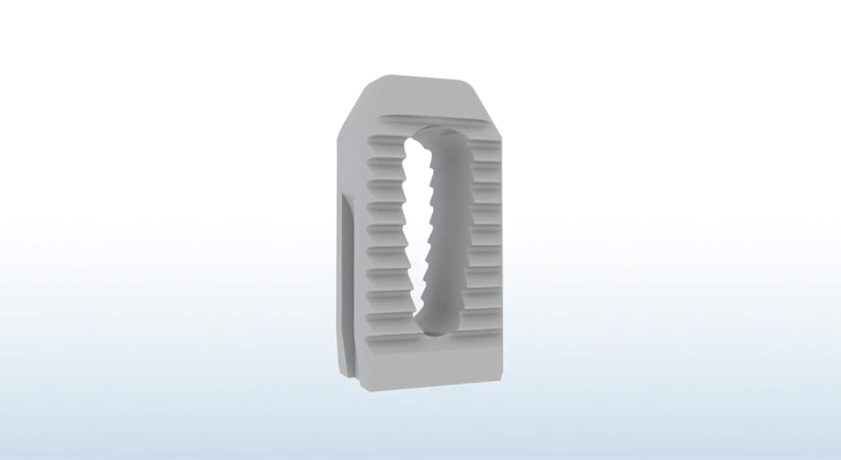
Final data from a landmark study on PainTEQ’s posterior approach SI joint fusion with the LinQ implant revealed improved pain and function scores and an excellent safety profile at 12 months post-operation.
The Single Arm, Multicenter, Prospective, Clinical Study on a Minimally Invasive Posterior Sacroiliac Fusion Allograft Implant (SECURE) study represents the largest and first-of-its-kind clinical trial for any posterior approach, and addresses the paucity of data for 12-month prospective outcomes. The study’s data on 83 patients revealed a statistically significant mean improvement in VAS scores of 43.3 at 12 months versus baseline. There was also a strong safety profile with only five total adverse events and no implant-related significant adverse events were observed.
In addition to the significant improvement in VAS scores, SECURE also showed statistically significant improvements in ODI scores, a measure of a patient’s perceived disability due to pain. Finally, the study found significant improvement in all PROMIS-29 domains, which represent pain intensity, sleep disturbance, fatigue, anxiety, depression, ability to participate in social roles and activities, and physical function.
SECURE marks the eighth publication directly related to the study of the LinQ implant itself.
“We are very encouraged by the results from the SECURE study,” said Sean LaNeve, PainTEQ’s CEO. “The 12-month data proves what our physicians and patients are experiencing and sharing with us on a consistent basis. The principles of medicine are to always exhaust the safest, least-invasive options first, and we believe SECURE proves that LinQ is a valid and valuable option for patients and physicians to consider before advancing to more invasive SI joint fusion devices.”
Source: PainTEQ
Final data from a landmark study on PainTEQ’s posterior approach SI joint fusion with the LinQ implant revealed improved pain and function scores and an excellent safety profile at 12 months post-operation.
The Single Arm, Multicenter, Prospective, Clinical Study on a Minimally Invasive Posterior Sacroiliac Fusion Allograft Implant (SECURE)...
Final data from a landmark study on PainTEQ’s posterior approach SI joint fusion with the LinQ implant revealed improved pain and function scores and an excellent safety profile at 12 months post-operation.
The Single Arm, Multicenter, Prospective, Clinical Study on a Minimally Invasive Posterior Sacroiliac Fusion Allograft Implant (SECURE) study represents the largest and first-of-its-kind clinical trial for any posterior approach, and addresses the paucity of data for 12-month prospective outcomes. The study’s data on 83 patients revealed a statistically significant mean improvement in VAS scores of 43.3 at 12 months versus baseline. There was also a strong safety profile with only five total adverse events and no implant-related significant adverse events were observed.
In addition to the significant improvement in VAS scores, SECURE also showed statistically significant improvements in ODI scores, a measure of a patient’s perceived disability due to pain. Finally, the study found significant improvement in all PROMIS-29 domains, which represent pain intensity, sleep disturbance, fatigue, anxiety, depression, ability to participate in social roles and activities, and physical function.
SECURE marks the eighth publication directly related to the study of the LinQ implant itself.
“We are very encouraged by the results from the SECURE study,” said Sean LaNeve, PainTEQ’s CEO. “The 12-month data proves what our physicians and patients are experiencing and sharing with us on a consistent basis. The principles of medicine are to always exhaust the safest, least-invasive options first, and we believe SECURE proves that LinQ is a valid and valuable option for patients and physicians to consider before advancing to more invasive SI joint fusion devices.”
Source: PainTEQ

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







