
 Copy to clipboard
Copy to clipboard 
FH Orthopedics marked the sale of >6,000 units of its cervical and lumbar artificial discs, CP-ESP® and LP-ESP®.
ESP discs comprise hydroxyapatite-coated titanium endplates surrounding a polymer elastomeric cushion (the Elastic Spine Pad), and are designed to mimic the natural anatomy in which bony segments are connected by a spongy disc.
LP-ESP received CE Mark approval in 2004, while CP-ESP received the regulatory approval in 2012 and launched in 2014.
Source: FH Orthopedics
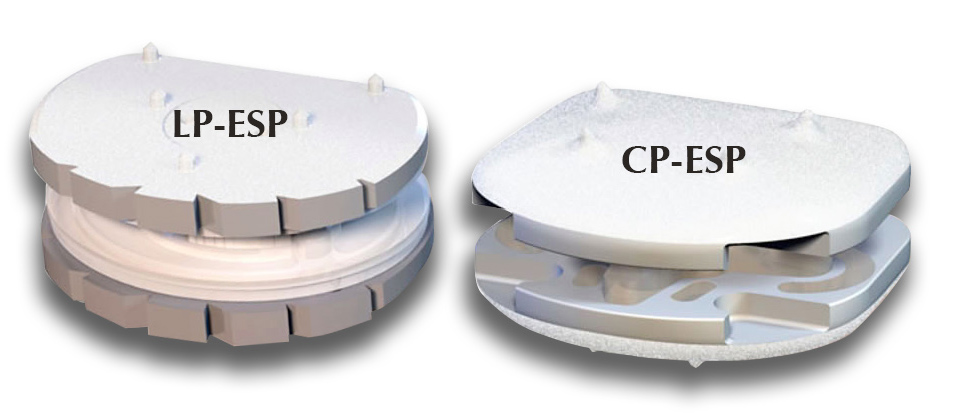
Image courtesy of FH Orthopedics
FH Orthopedics marked the sale of >6,000 units of its cervical and lumbar artificial discs, CP-ESP® and LP-ESP®.
ESP discs comprise hydroxyapatite-coated titanium endplates surrounding a polymer elastomeric cushion (the Elastic Spine Pad), and are designed to mimic the natural anatomy in which bony segments are connected by a spongy...
FH Orthopedics marked the sale of >6,000 units of its cervical and lumbar artificial discs, CP-ESP® and LP-ESP®.
ESP discs comprise hydroxyapatite-coated titanium endplates surrounding a polymer elastomeric cushion (the Elastic Spine Pad), and are designed to mimic the natural anatomy in which bony segments are connected by a spongy disc.
LP-ESP received CE Mark approval in 2004, while CP-ESP received the regulatory approval in 2012 and launched in 2014.
Source: FH Orthopedics

Image courtesy of FH Orthopedics

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







