
 Copy to clipboard
Copy to clipboard 
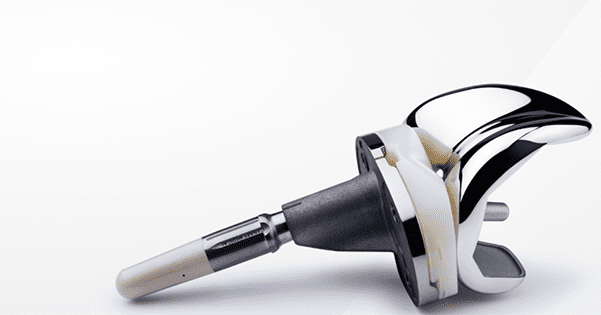
Zimmer Biomet and Canary Medical announced the FDA De Novo classification grant and authorization to market the tibial extension for Persona IQ®, the first and only smart knee cleared by FDA for total knee replacement. Persona IQ combines Zimmer Biomet’s Persona® The Personalized Knee® with Canary Medical’s implantable canturio™ te tibial extension sensor technology that measures and determines range of motion, step count, walking speed and other gait metrics. Persona IQ will work with Zimmer Biomet’s remote care management platform, mymobility® with Apple Watch®, as well as other components of the ZBEdge™ Connected Intelligence Suite.
Once surgically implanted in the knee, Persona IQ records and wirelessly transmits a wide range of gait data to a patient’s personal base station at home. The data are then securely delivered to a cloud-based platform. Surgeons can assess post-surgery recovery progress by comparing pre-operative mobility metrics captured by mymobility, with post-operative gait metrics collected by Persona IQ. Ultimately, the data from Persona IQ will be seamlessly integrated with pre-, intra- and post-operative data collected by the ZBEdge Connected Intelligence Suite and analyzed by OrthoIntel Orthopedic Intelligence Platform to provide surgeons with objective data as a supplement to patient care.
Persona IQ features the CANARY canturio te tibial extension proprietary implantable sensor developed by Canary Medical. The sensor technology was licensed by Zimmer Biomet through an exclusive partnership agreement with Canary Medical to develop first-to-market smart orthopedic implants to support remote monitoring and tracking of patient recovery metrics.
The CANARY canturio te uses the same material and technology found in implanted cardiac devices such as pacemakers. The sensor is powered by a battery with a lifespan of up to 10 years, so patients will not need to charge the device. In addition, as an implanted technology, data is collected passively and does not rely on daily patient compliance to ensure information is captured by Persona IQ.
Persona IQ will be available to healthcare professionals and patients in the coming months.
Source: Zimmer Biomet
Zimmer Biomet and Canary Medical announced the FDA De Novo classification grant and authorization to market the tibial extension for Persona IQ®, the first and only smart knee cleared by FDA for total knee replacement. Persona IQ combines Zimmer Biomet's Persona® The Personalized Knee® with Canary Medical's implantable canturio™ te tibial...
Zimmer Biomet and Canary Medical announced the FDA De Novo classification grant and authorization to market the tibial extension for Persona IQ®, the first and only smart knee cleared by FDA for total knee replacement. Persona IQ combines Zimmer Biomet’s Persona® The Personalized Knee® with Canary Medical’s implantable canturio™ te tibial extension sensor technology that measures and determines range of motion, step count, walking speed and other gait metrics. Persona IQ will work with Zimmer Biomet’s remote care management platform, mymobility® with Apple Watch®, as well as other components of the ZBEdge™ Connected Intelligence Suite.
Once surgically implanted in the knee, Persona IQ records and wirelessly transmits a wide range of gait data to a patient’s personal base station at home. The data are then securely delivered to a cloud-based platform. Surgeons can assess post-surgery recovery progress by comparing pre-operative mobility metrics captured by mymobility, with post-operative gait metrics collected by Persona IQ. Ultimately, the data from Persona IQ will be seamlessly integrated with pre-, intra- and post-operative data collected by the ZBEdge Connected Intelligence Suite and analyzed by OrthoIntel Orthopedic Intelligence Platform to provide surgeons with objective data as a supplement to patient care.
Persona IQ features the CANARY canturio te tibial extension proprietary implantable sensor developed by Canary Medical. The sensor technology was licensed by Zimmer Biomet through an exclusive partnership agreement with Canary Medical to develop first-to-market smart orthopedic implants to support remote monitoring and tracking of patient recovery metrics.
The CANARY canturio te uses the same material and technology found in implanted cardiac devices such as pacemakers. The sensor is powered by a battery with a lifespan of up to 10 years, so patients will not need to charge the device. In addition, as an implanted technology, data is collected passively and does not rely on daily patient compliance to ensure information is captured by Persona IQ.
Persona IQ will be available to healthcare professionals and patients in the coming months.
Source: Zimmer Biomet

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







