
 Copy to clipboard
Copy to clipboard 
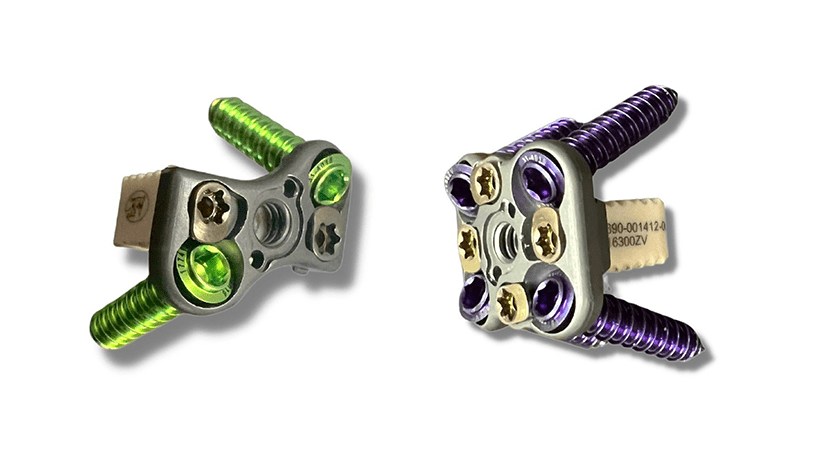
Zavation Medical Products was granted FDA 510(k) clearance to market Varisync™, a cervical intervertebral body fusion device.
Varisync has been tested and approved for both the independent and synchronized use of its plate and spacer components. This allows surgeons to use these plate and spacer options together as a system or pair individual components with any of Zavation’s existing cervical portfolio options. The Varisync plate features visual and tactile confirmation of locks, ideal screw angulation, multiple insertion devices and optimal spacer placement in the disc space.
The VariSync Plate is intended for anterior screw fixation to the cervical spine for the treatment of degenerative disc disease, trauma, tumors, deformity, pseudarthrosis, failed previous fusion, spondylolisthesis and spinal stenosis.
The VariSync Spacer is an interbody fusion device intended to treat degenerative disc disease of the cervical spine at one level. The spacer is to be filled with autograft or allogenic bone graft comprising cancellous and/or corticancellous bone graft in skeletally mature patients. These devices are intended to be used with supplemental fixation such as the Zavation VariSync Plate, Zavation Midline Plate, Zavation EZ Plate or Zavation Cervical Plate Systems. When used with the VariSync Plate, the assembly takes on the indications of the VariSync Spacer, with the VariSync Plate acting as the supplemental fixation.
Source: Zavation Medical Products
Zavation Medical Products was granted FDA 510(k) clearance to market Varisync™, a cervical intervertebral body fusion device.
Varisync has been tested and approved for both the independent and synchronized use of its plate and spacer components. This allows surgeons to use these plate and spacer options together as a system or pair individual...
Zavation Medical Products was granted FDA 510(k) clearance to market Varisync™, a cervical intervertebral body fusion device.
Varisync has been tested and approved for both the independent and synchronized use of its plate and spacer components. This allows surgeons to use these plate and spacer options together as a system or pair individual components with any of Zavation’s existing cervical portfolio options. The Varisync plate features visual and tactile confirmation of locks, ideal screw angulation, multiple insertion devices and optimal spacer placement in the disc space.
The VariSync Plate is intended for anterior screw fixation to the cervical spine for the treatment of degenerative disc disease, trauma, tumors, deformity, pseudarthrosis, failed previous fusion, spondylolisthesis and spinal stenosis.
The VariSync Spacer is an interbody fusion device intended to treat degenerative disc disease of the cervical spine at one level. The spacer is to be filled with autograft or allogenic bone graft comprising cancellous and/or corticancellous bone graft in skeletally mature patients. These devices are intended to be used with supplemental fixation such as the Zavation VariSync Plate, Zavation Midline Plate, Zavation EZ Plate or Zavation Cervical Plate Systems. When used with the VariSync Plate, the assembly takes on the indications of the VariSync Spacer, with the VariSync Plate acting as the supplemental fixation.
Source: Zavation Medical Products

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







