
 Copy to clipboard
Copy to clipboard 
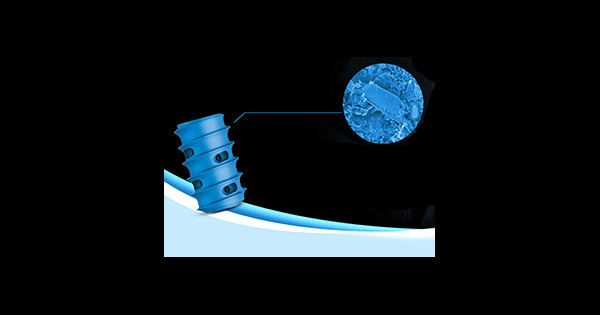
SurGenTec received FDA clearance to market its proprietary standalone spine fixation implant: ION, the smallest posterior fixation implant cleared for spine surgery. This device can be used to treat a variety of pathologies throughout the spine from C2-S1.
ION screws are enhanced with Nanotex™ surface technology, which was designed with bony integration in mind. The implant is approximately half the size of a dime and can be placed through a tiny incision with minimal tissue disruption. ION provides patients with a treatment option between conservative care and major interbody fusion in the algorithm of care.
ION can be used in a variety of open or true minimally invasive surgery (MIS) applications including facet fusions, adjunct to decompressive procedures, the treatment of adjacent level disease or to provide added stability with interbody fusions. The ION facet screw system instruments were designed to minimize tissue trauma and incision size while delivering the ION implants to the surgical location.
Unlike other spinal fixation options, ION has zero profile above the bone, which may prevent soft tissue contact and the potential for irritation. The implants may be packed with bone graft prior to and after insertion to help facilitate fusion while providing an opportunity for bone to bridge over the implant and prevent implant migration. ION also comes in an array of sizes to match different patient anatomy.
Source: SurGenTec
SurGenTec received FDA clearance to market its proprietary standalone spine fixation implant: ION, the smallest posterior fixation implant cleared for spine surgery. This device can be used to treat a variety of pathologies throughout the spine from C2-S1.
ION screws are enhanced with Nanotex™ surface technology, which was designed with bony...
SurGenTec received FDA clearance to market its proprietary standalone spine fixation implant: ION, the smallest posterior fixation implant cleared for spine surgery. This device can be used to treat a variety of pathologies throughout the spine from C2-S1.
ION screws are enhanced with Nanotex™ surface technology, which was designed with bony integration in mind. The implant is approximately half the size of a dime and can be placed through a tiny incision with minimal tissue disruption. ION provides patients with a treatment option between conservative care and major interbody fusion in the algorithm of care.
ION can be used in a variety of open or true minimally invasive surgery (MIS) applications including facet fusions, adjunct to decompressive procedures, the treatment of adjacent level disease or to provide added stability with interbody fusions. The ION facet screw system instruments were designed to minimize tissue trauma and incision size while delivering the ION implants to the surgical location.
Unlike other spinal fixation options, ION has zero profile above the bone, which may prevent soft tissue contact and the potential for irritation. The implants may be packed with bone graft prior to and after insertion to help facilitate fusion while providing an opportunity for bone to bridge over the implant and prevent implant migration. ION also comes in an array of sizes to match different patient anatomy.
Source: SurGenTec

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







