
 Copy to clipboard
Copy to clipboard 
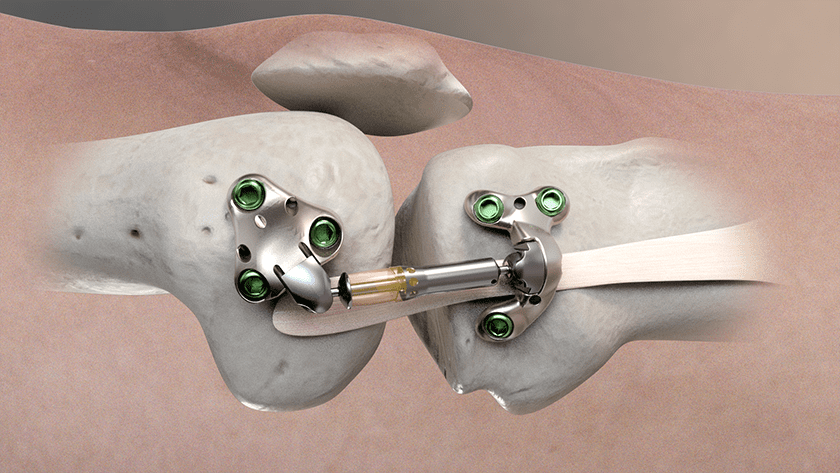
FDA granted marketing authorization of Moximed’s MISHA Knee, an implantable shock absorber (ISA) for the knee. The MISHA Knee system is indicated to treat people with medial knee osteoarthritis (OA) who failed to find relief from non-surgical or surgical treatment, continue to experience pain that interferes with daily activities, and are ineligible for, or unwilling to undergo, joint replacement due to age or absence of advanced OA.
Moximed utilized the clinically established benefits of load reduction on diseased joints to design the MISHA Knee. Implanted during an outpatient-compatible procedure, the system demonstrated superiority over high tibial osteotomy, a well-established surgery with decades of demonstrated clinical results, in its recent pivotal clinical study.
“This is a milestone event for knee OA sufferers, and it’s the result of unwavering clinical research and development that spans more than 10 years,” said Anton Clifford, Ph.D., founder and CEO of Moximed. “We are committed to providing excellent medical education and customer service, supporting selection and treatment of indicated patients, and demonstrating scalability of our business as we introduce the MISHA Knee System to the U.S.”
Source: Moximed
FDA granted marketing authorization of Moximed's MISHA Knee, an implantable shock absorber (ISA) for the knee. The MISHA Knee system is indicated to treat people with medial knee osteoarthritis (OA) who failed to find relief from non-surgical or surgical treatment, continue to experience pain that interferes with daily activities, and are...
FDA granted marketing authorization of Moximed’s MISHA Knee, an implantable shock absorber (ISA) for the knee. The MISHA Knee system is indicated to treat people with medial knee osteoarthritis (OA) who failed to find relief from non-surgical or surgical treatment, continue to experience pain that interferes with daily activities, and are ineligible for, or unwilling to undergo, joint replacement due to age or absence of advanced OA.
Moximed utilized the clinically established benefits of load reduction on diseased joints to design the MISHA Knee. Implanted during an outpatient-compatible procedure, the system demonstrated superiority over high tibial osteotomy, a well-established surgery with decades of demonstrated clinical results, in its recent pivotal clinical study.
“This is a milestone event for knee OA sufferers, and it’s the result of unwavering clinical research and development that spans more than 10 years,” said Anton Clifford, Ph.D., founder and CEO of Moximed. “We are committed to providing excellent medical education and customer service, supporting selection and treatment of indicated patients, and demonstrating scalability of our business as we introduce the MISHA Knee System to the U.S.”
Source: Moximed

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







