
 Copy to clipboard
Copy to clipboard 
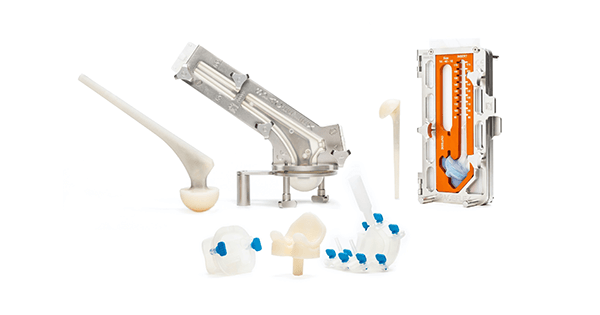
G21 was granted FDA 510(k) clearance to market the SpaceFlex Shoulder™, completing its Spaceflex line that includes hip and knee spacers.
“The achievement of this result for G21® S.r.l. means being able to offer a dedicated treatment of each infected joint,” said Filippo Foroni, Executive Vice President. “Since we set up the company in 2009, we are committed to provide our patients with better health conditions. The addition of SpaceFlex Shoulder™ to our product portfolio represents a user-friendly solution for complex issues.”
G21 provides bone cement for orthopedics with different levels of viscosity for the making of temporary prothesis for the treatment of infections and septic revisions. Earlier this year, the company completed its line of custom modular spacers with the introduction of the SpaceFlex r-evolution™ trials kit line to treat infection.
G21 was granted FDA 510(k) clearance to market the SpaceFlex Shoulder™, completing its Spaceflex line that includes hip and knee spacers.
"The achievement of this result for G21® S.r.l. means being able to offer a dedicated treatment of each infected joint," said Filippo Foroni, Executive Vice President. "Since we set up the company in...
G21 was granted FDA 510(k) clearance to market the SpaceFlex Shoulder™, completing its Spaceflex line that includes hip and knee spacers.
“The achievement of this result for G21® S.r.l. means being able to offer a dedicated treatment of each infected joint,” said Filippo Foroni, Executive Vice President. “Since we set up the company in 2009, we are committed to provide our patients with better health conditions. The addition of SpaceFlex Shoulder™ to our product portfolio represents a user-friendly solution for complex issues.”
G21 provides bone cement for orthopedics with different levels of viscosity for the making of temporary prothesis for the treatment of infections and septic revisions. Earlier this year, the company completed its line of custom modular spacers with the introduction of the SpaceFlex r-evolution™ trials kit line to treat infection.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







