
 Copy to clipboard
Copy to clipboard 
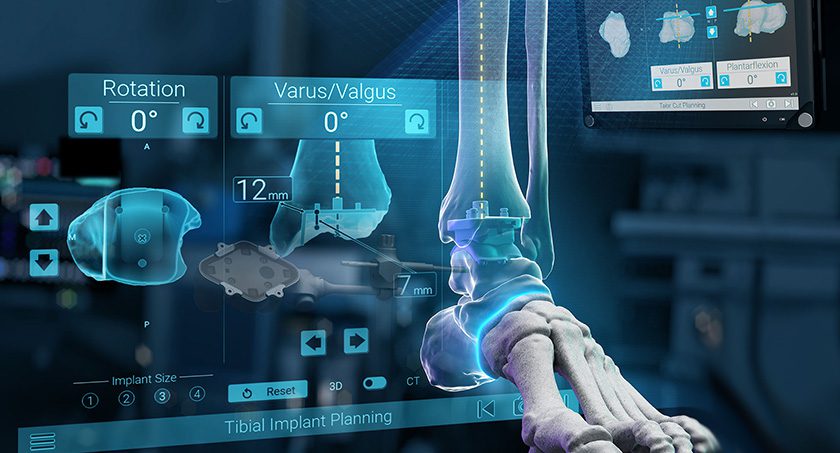
Exactech subsidiary, BlueOrtho, received FDA 510(k) clearance to market ExactechGPS Ankle, reported to be the world’s first surgical navigation system for total ankle replacement.
GPS Ankle is only available in the U.S. and will enter pilot launch with limited availability starting in 2024.
GPS Ankle connects the preoperative plan with real-time intraoperative instrument guidance and confirms that resections meet the surgical plan. The system uses proprietary active tracker technology and a compact touchscreen tablet in the sterile field to provide surgeons with dynamic intraoperative feedback throughout their cases.
GPS Ankle is compatible with Exactech’s Vantage Total Ankle and will be available to hospitals and ASCs without capital cost. Pre-clinical studies, based on bench testing, reported an accuracy of 2mm and 2 degrees relative to the CT-based surgical plan. This was further confirmed by two studies performed on sawbones.
“Exactech has introduced new concepts in the ankle market, but this clearance represents the first intraoperative guidance for ankle surgery and solidifies us as a company focused on innovation,” said Laurent Angibaud, Exactech’s Vice President of Development for Advanced Surgical Technologies.
Source: Exactech, Inc.
Exactech subsidiary, BlueOrtho, received FDA 510(k) clearance to market ExactechGPS Ankle, reported to be the world’s first surgical navigation system for total ankle replacement.
GPS Ankle is only available in the U.S. and will enter pilot launch with limited availability starting in 2024.
GPS Ankle connects the preoperative plan with...
Exactech subsidiary, BlueOrtho, received FDA 510(k) clearance to market ExactechGPS Ankle, reported to be the world’s first surgical navigation system for total ankle replacement.
GPS Ankle is only available in the U.S. and will enter pilot launch with limited availability starting in 2024.
GPS Ankle connects the preoperative plan with real-time intraoperative instrument guidance and confirms that resections meet the surgical plan. The system uses proprietary active tracker technology and a compact touchscreen tablet in the sterile field to provide surgeons with dynamic intraoperative feedback throughout their cases.
GPS Ankle is compatible with Exactech’s Vantage Total Ankle and will be available to hospitals and ASCs without capital cost. Pre-clinical studies, based on bench testing, reported an accuracy of 2mm and 2 degrees relative to the CT-based surgical plan. This was further confirmed by two studies performed on sawbones.
“Exactech has introduced new concepts in the ankle market, but this clearance represents the first intraoperative guidance for ankle surgery and solidifies us as a company focused on innovation,” said Laurent Angibaud, Exactech’s Vice President of Development for Advanced Surgical Technologies.
Source: Exactech, Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







