
 Copy to clipboard
Copy to clipboard 
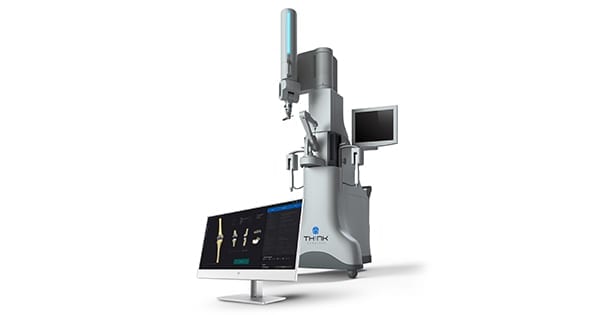
THINK Surgical and Ortho Development announced FDA clearance for use of Ortho Development’s Balanced Knee® System (BKS) and BKS TriMax® implants with THINK Surgical’s TSolution One® Total Knee Application. TSolution One is the only commercially available active robot system for total knee replacement that supports an open implant library.
The Balanced Knee System and BKS TriMax includes implants and instrumentation for total knee replacement. TSolution One comprises TPLAN®, the 3D pre-surgical workstation, and TCAT®, the active robot. TSolution One supports optimal implant placement based on each patient’s anatomy.
Ortho Development’s BKS and BKS TriMax implants feature a locking mechanism specifically designed to minimize micro-motion between the plastic tibial insert and the titanium tibial base plate implants.
Brent Bartholomew, President of Ortho Development, said, “The BKS and BKS TriMax have an excellent clinical history and market reputation going back 20+ years. Coupling these implants with the TSolution One Total Knee Application adds a unique enabling technology for our flagship products.”
THINK Surgical and Ortho Development announced FDA clearance for use of Ortho Development's Balanced Knee® System (BKS) and BKS TriMax® implants with THINK Surgical's TSolution One® Total Knee Application. TSolution One is the only commercially available active robot system for total knee replacement that supports an open implant library.
...
THINK Surgical and Ortho Development announced FDA clearance for use of Ortho Development’s Balanced Knee® System (BKS) and BKS TriMax® implants with THINK Surgical’s TSolution One® Total Knee Application. TSolution One is the only commercially available active robot system for total knee replacement that supports an open implant library.
The Balanced Knee System and BKS TriMax includes implants and instrumentation for total knee replacement. TSolution One comprises TPLAN®, the 3D pre-surgical workstation, and TCAT®, the active robot. TSolution One supports optimal implant placement based on each patient’s anatomy.
Ortho Development’s BKS and BKS TriMax implants feature a locking mechanism specifically designed to minimize micro-motion between the plastic tibial insert and the titanium tibial base plate implants.
Brent Bartholomew, President of Ortho Development, said, “The BKS and BKS TriMax have an excellent clinical history and market reputation going back 20+ years. Coupling these implants with the TSolution One Total Knee Application adds a unique enabling technology for our flagship products.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







