
 Copy to clipboard
Copy to clipboard 
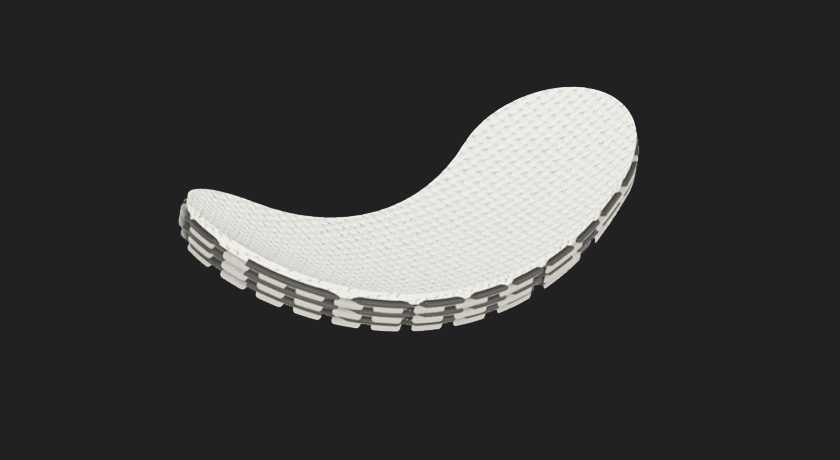
FDA approved a Phase I clinical trial, allowing CytexOrtho to initiate a first-in-human clinical study to evaluate the safety and efficacy of its ReNew Hip Implant novel cartilage repair technology.
The proprietary ReNew Hip Implant leverages advanced manufacturing techniques to create a highly porous, bioabsorbable device engineered to mimic the properties of healthy articular cartilage. In animal trials, ReNew Hip demonstrated immediate structural support while supporting the body’s own regenerative, healing processes to restore the joint.
CytexOrtho will enroll up to 15 patients aged 14-55 years with hip disease resulting in loss of articular cartilage integrity on the femoral head. The non-randomized, single-arm study will establish initial safety profile and evaluate efficacy in improving pain and function over 12 months. It will follow patients for 60 months post-implantation.
“There are over one million American patients under the age of 65 who suffer with chronic hip pain,” said Brad Estes, Ph.D., CEO and Co-founder, CytexOrtho. “Approximately 20 percent of these patients get hip replacements. The rest avoid them because of the high risk of wearing them out and the complications that come with a revision replacement, and instead choose to live with increasingly crippling pain. FDA’s approval of this first human clinical trial brings us closer to delivering new options to the clinic for patients with hip disease. Our ReNew™ Hip Implant aims to change the game by restoring the joint’s anatomical contour with natural tissue regeneration.”
Source: CytexOrtho
FDA approved a Phase I clinical trial, allowing CytexOrtho to initiate a first-in-human clinical study to evaluate the safety and efficacy of its ReNew Hip Implant novel cartilage repair technology.
The proprietary ReNew Hip Implant leverages advanced manufacturing techniques to create a highly porous, bioabsorbable device engineered to mimic...
FDA approved a Phase I clinical trial, allowing CytexOrtho to initiate a first-in-human clinical study to evaluate the safety and efficacy of its ReNew Hip Implant novel cartilage repair technology.
The proprietary ReNew Hip Implant leverages advanced manufacturing techniques to create a highly porous, bioabsorbable device engineered to mimic the properties of healthy articular cartilage. In animal trials, ReNew Hip demonstrated immediate structural support while supporting the body’s own regenerative, healing processes to restore the joint.
CytexOrtho will enroll up to 15 patients aged 14-55 years with hip disease resulting in loss of articular cartilage integrity on the femoral head. The non-randomized, single-arm study will establish initial safety profile and evaluate efficacy in improving pain and function over 12 months. It will follow patients for 60 months post-implantation.
“There are over one million American patients under the age of 65 who suffer with chronic hip pain,” said Brad Estes, Ph.D., CEO and Co-founder, CytexOrtho. “Approximately 20 percent of these patients get hip replacements. The rest avoid them because of the high risk of wearing them out and the complications that come with a revision replacement, and instead choose to live with increasingly crippling pain. FDA’s approval of this first human clinical trial brings us closer to delivering new options to the clinic for patients with hip disease. Our ReNew™ Hip Implant aims to change the game by restoring the joint’s anatomical contour with natural tissue regeneration.”
Source: CytexOrtho

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







