
 Copy to clipboard
Copy to clipboard 
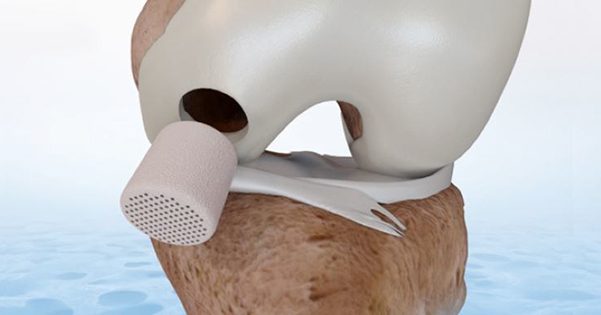
FDA granted premarket approval for CartiHeal’s Agili-C implant™ for the treatment of knee joint surface lesions.
The approval was granted based on results of a two-year Investigational Device Exemption pivotal clinical study which confirmed superiority of the Agili-C implant over the current surgical standard of care (SSOC), microfracture and debridement, for the treatment of knee joint surface lesions, chondral and osteochondral defects. The study was multicenter, 2:1 randomization, open-labeled and controlled. A total of 251 subjects were enrolled: 167 in the Agili-C arm and 84 in the SSOC arm, in 26 sites in and outside the U.S.
CartiHeal was granted a Breakthrough Device Designation for Agili-C in 2020. In 2021, Bioventus made a $50 million escrow payment pursuant to its Option and Equity Purchase Agreement with CartiHeal, signaling its intent to move forward with an acquisition of CartiHeal.
“The 2-year study results, which demonstrated superiority of the Agili-C™ implant over the current surgical standard of care, offers an important potential benefit to millions of patients,” said Nir Altschuler, CartiHeal’s founder and CEO. “This milestone achievement was made possible due to the support of our regulatory advisors, Hogan Lovells, our statistical consultants, Biomedical Statistical Consulting, and the many dedicated investigators and patients who participated in our studies. We are grateful for all their help. FDA’s approval enables us to initiate commercialization and provide a superior solution for patients compared to the current standard of care options.”
Source: CartiHeal
FDA granted premarket approval for CartiHeal's Agili-C implant™ for the treatment of knee joint surface lesions.
The approval was granted based on results of a two-year Investigational Device Exemption pivotal clinical study which confirmed superiority of the Agili-C implant over the current surgical standard of care (SSOC), microfracture and...
FDA granted premarket approval for CartiHeal’s Agili-C implant™ for the treatment of knee joint surface lesions.
The approval was granted based on results of a two-year Investigational Device Exemption pivotal clinical study which confirmed superiority of the Agili-C implant over the current surgical standard of care (SSOC), microfracture and debridement, for the treatment of knee joint surface lesions, chondral and osteochondral defects. The study was multicenter, 2:1 randomization, open-labeled and controlled. A total of 251 subjects were enrolled: 167 in the Agili-C arm and 84 in the SSOC arm, in 26 sites in and outside the U.S.
CartiHeal was granted a Breakthrough Device Designation for Agili-C in 2020. In 2021, Bioventus made a $50 million escrow payment pursuant to its Option and Equity Purchase Agreement with CartiHeal, signaling its intent to move forward with an acquisition of CartiHeal.
“The 2-year study results, which demonstrated superiority of the Agili-C™ implant over the current surgical standard of care, offers an important potential benefit to millions of patients,” said Nir Altschuler, CartiHeal’s founder and CEO. “This milestone achievement was made possible due to the support of our regulatory advisors, Hogan Lovells, our statistical consultants, Biomedical Statistical Consulting, and the many dedicated investigators and patients who participated in our studies. We are grateful for all their help. FDA’s approval enables us to initiate commercialization and provide a superior solution for patients compared to the current standard of care options.”
Source: CartiHeal

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







