
 Copy to clipboard
Copy to clipboard 
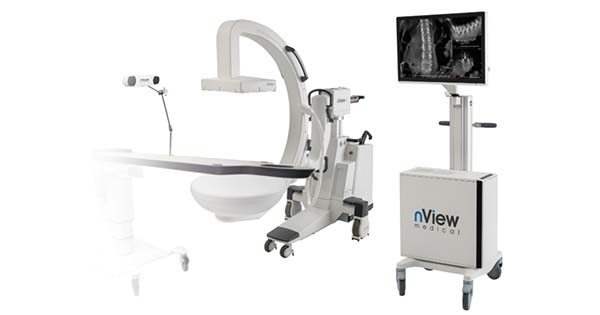
nView medical announces FDA 510(k) clearance of the nView s1 with navigation option.
nView s1 is an imaging system that integrates the latest developments in low-dose X-ray imaging and AI-enabled imaging algorithms to provide multiplanar, fluoroscopic and augmented views derived from fast tomographic reconstructions of the patient during surgery. With the addition of navigation these images can now be used for surgical guidance, providing greater utility for surgeons during spine procedures.
nView medical CEO, Cristian Atria, commented: “What makes nView’s approach a breakthrough is that with insta-3D™, 3D images can be taken on the fly during surgery, providing a true representation of the anatomy. These images can be navigated immediately, we call this unique approach true-map navigation™. We believe that the nView s1 with integrated navigation is the most efficient solution in terms of minimizing surgical time, minimizing the footprint in the OR, and minimizing radiation to the patient. This is a major milestone for nView as we augment our image creation offering with image utilization technologies. With this 510(k) we also cleared AI-enabled image analysis and advanced imaging modes such as 3D stitching.”
Source: nView medical
nView medical announces FDA 510(k) clearance of the nView s1 with navigation option.
nView s1 is an imaging system that integrates the latest developments in low-dose X-ray imaging and AI-enabled imaging algorithms to provide multiplanar, fluoroscopic and augmented views derived from fast tomographic reconstructions of the patient during...
nView medical announces FDA 510(k) clearance of the nView s1 with navigation option.
nView s1 is an imaging system that integrates the latest developments in low-dose X-ray imaging and AI-enabled imaging algorithms to provide multiplanar, fluoroscopic and augmented views derived from fast tomographic reconstructions of the patient during surgery. With the addition of navigation these images can now be used for surgical guidance, providing greater utility for surgeons during spine procedures.
nView medical CEO, Cristian Atria, commented: “What makes nView’s approach a breakthrough is that with insta-3D™, 3D images can be taken on the fly during surgery, providing a true representation of the anatomy. These images can be navigated immediately, we call this unique approach true-map navigation™. We believe that the nView s1 with integrated navigation is the most efficient solution in terms of minimizing surgical time, minimizing the footprint in the OR, and minimizing radiation to the patient. This is a major milestone for nView as we augment our image creation offering with image utilization technologies. With this 510(k) we also cleared AI-enabled image analysis and advanced imaging modes such as 3D stitching.”
Source: nView medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







