
 Copy to clipboard
Copy to clipboard 
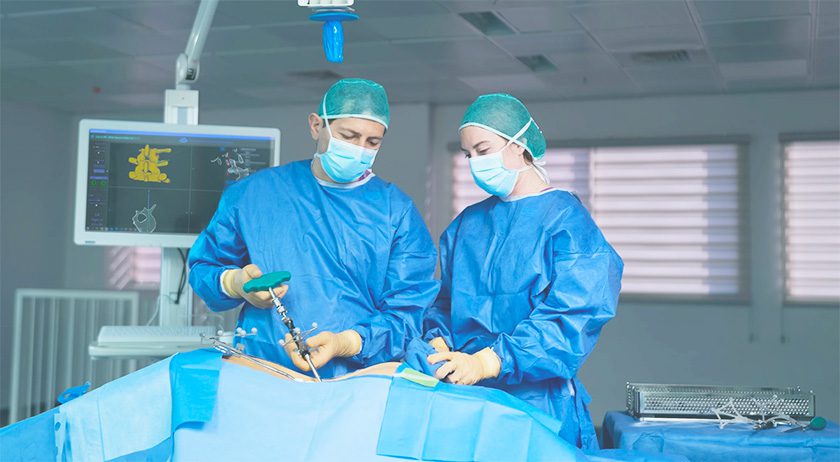
PathKeeper Surgical received FDA 510(k) clearance to market the PathKeeper 3D optical navigation system.
PathKeeper was designed to replace traditional navigation technology with a 3D optical navigation system that offers active, real-time, independent tracking of patient anatomy and surgical instruments, accuracy of implantation to less than a millimeter, more efficient surgical workflow, elimination of radiation exposure during the surgical procedure and a more economical price so both hospital and ambulatory-surgical center operating rooms can incorporate this new technology.
The device is indicated for posterior approach open spine fusion surgery during pedicle screw placement in the thoracolumbo-sacral region where reference to a rigid anatomical structure can be identified.
Source: PathKeeper Surgical
PathKeeper Surgical received FDA 510(k) clearance to market the PathKeeper 3D optical navigation system.
PathKeeper was designed to replace traditional navigation technology with a 3D optical navigation system that offers active, real-time, independent tracking of patient anatomy and surgical instruments, accuracy of implantation to less than...
PathKeeper Surgical received FDA 510(k) clearance to market the PathKeeper 3D optical navigation system.
PathKeeper was designed to replace traditional navigation technology with a 3D optical navigation system that offers active, real-time, independent tracking of patient anatomy and surgical instruments, accuracy of implantation to less than a millimeter, more efficient surgical workflow, elimination of radiation exposure during the surgical procedure and a more economical price so both hospital and ambulatory-surgical center operating rooms can incorporate this new technology.
The device is indicated for posterior approach open spine fusion surgery during pedicle screw placement in the thoracolumbo-sacral region where reference to a rigid anatomical structure can be identified.
Source: PathKeeper Surgical

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.






