
 Copy to clipboard
Copy to clipboard 
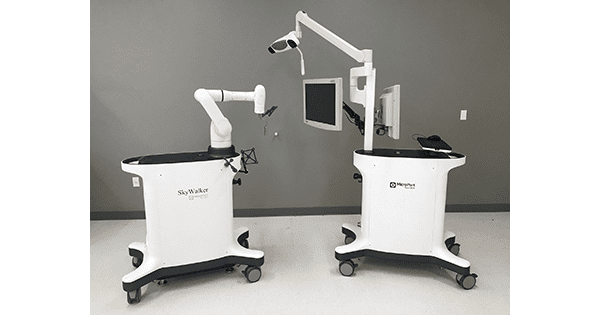
MicroPort Navibot received FDA 510(K) clearance to market SkyWalker™, the company’s first robot-assisted platform for orthopedic applications. SkyWalker will initially offer a robotically-assisted total knee replacement solution that is compatible with the Evolution® Medial-Pivot Total Knee.
The system was used in its first-in-man case in 2020.
MicroPort NaviBot has built a full technology research facility that has medical industry design capability, and benefits from having a self-developed, high-dexterity and lightweight mechanical arm combined with planning and navigation algorithms.
Prior to surgery, the planning system assists surgeons in formulating personalized patient implant plans based on preoperative CT scan anatomical data as well as specific implant data. During the procedure, SkyWalker facilitates precise implant positioning and allows the surgeon to quickly proceed to resection.
The SkyWalker System is capable of providing the surgeon with information that can help to achieve the desired joint line reconstruction while providing data to optimally balance soft tissues. MicroPort Navibot is planning to develop other orthopedic applications in the near future, in conjunction with MicroPort Orthopedics.
Source: MicroPort Orthopedics
MicroPort Navibot received FDA 510(K) clearance to market SkyWalker™, the company's first robot-assisted platform for orthopedic applications. SkyWalker will initially offer a robotically-assisted total knee replacement solution that is compatible with the Evolution® Medial-Pivot Total Knee.
The system was used in its first-in-man case in...
MicroPort Navibot received FDA 510(K) clearance to market SkyWalker™, the company’s first robot-assisted platform for orthopedic applications. SkyWalker will initially offer a robotically-assisted total knee replacement solution that is compatible with the Evolution® Medial-Pivot Total Knee.
The system was used in its first-in-man case in 2020.
MicroPort NaviBot has built a full technology research facility that has medical industry design capability, and benefits from having a self-developed, high-dexterity and lightweight mechanical arm combined with planning and navigation algorithms.
Prior to surgery, the planning system assists surgeons in formulating personalized patient implant plans based on preoperative CT scan anatomical data as well as specific implant data. During the procedure, SkyWalker facilitates precise implant positioning and allows the surgeon to quickly proceed to resection.
The SkyWalker System is capable of providing the surgeon with information that can help to achieve the desired joint line reconstruction while providing data to optimally balance soft tissues. MicroPort Navibot is planning to develop other orthopedic applications in the near future, in conjunction with MicroPort Orthopedics.
Source: MicroPort Orthopedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







