
 Copy to clipboard
Copy to clipboard 
A new system for fixing bone fractures, called the Bone Bolt System, received FDA 510(k) marketing clearance as announced by the University of Utah Orthopaedic Innovation Center (OIC), Department of Orthopaedics and Spencer Fox Eccles School of Medicine.

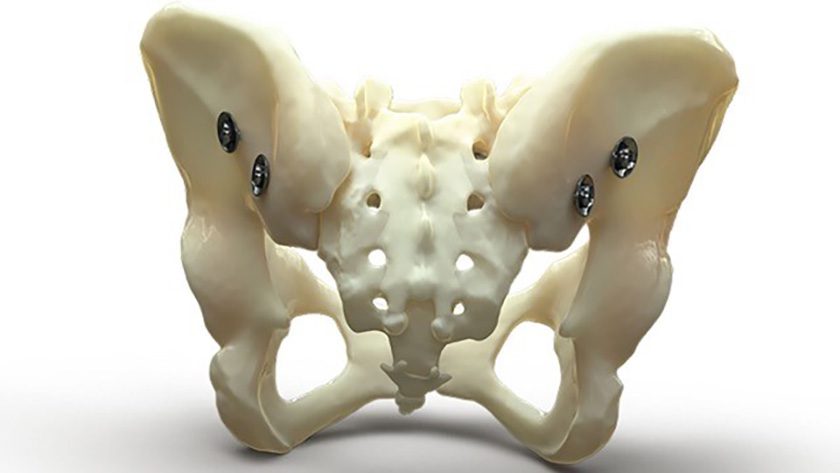
The Bone Bolt System
Bone Bolt is a novel implant designed and developed for percutaneous bone fracture fixation. The system comprises implants of various lengths and diameters, along with associated surgical instruments and sterilization trays. The implants are used to treat challenging bone fractures, such as pelvic fractures and fractures of the long bones in the arm and leg. Bone Bolt is protected by U.S. Patent No. 11,553,948, with other U.S. and international patents pending.
The OIC submitted a 510(k) to the FDA to demonstrate that the medical device is safe and effective, or “substantially equivalent,” to an existing FDA 510(k) cleared device. Upon 510(k) clearance by the FDA, the medical device may be legally marketed in the U.S.
Following this clearance, the OIC and the University of Utah PIVOT Center will establish industry partnerships to commercialize the Bone Bolt System and bring it to hospitals and surgery centers throughout the U.S.
“The simplicity of the Bone Bolt procedure to effectively stabilize complex fractures will impact the standard of care for patients with challenging fractures. The thoughtfulness during development of the Bone Bolt System encourages rapid adoption as the development team considered the care provider’s perspective in conjunction with clinical outcomes,” said PIVOT Associate Director of Innovation & Commercialization Huy Tran. “PIVOT Center is looking forward to engaging with potential industry partners to commercialize the technology and make an immediate impact on the quality of life for patients.”
Source: University of Utah Health
A new system for fixing bone fractures, called the Bone Bolt System, received FDA 510(k) marketing clearance as announced by the University of Utah Orthopaedic Innovation Center (OIC), Department of Orthopaedics and Spencer Fox Eccles School of Medicine.
Bone Bolt is a novel implant designed and developed for percutaneous bone fracture...
A new system for fixing bone fractures, called the Bone Bolt System, received FDA 510(k) marketing clearance as announced by the University of Utah Orthopaedic Innovation Center (OIC), Department of Orthopaedics and Spencer Fox Eccles School of Medicine.

The Bone Bolt System
Bone Bolt is a novel implant designed and developed for percutaneous bone fracture fixation. The system comprises implants of various lengths and diameters, along with associated surgical instruments and sterilization trays. The implants are used to treat challenging bone fractures, such as pelvic fractures and fractures of the long bones in the arm and leg. Bone Bolt is protected by U.S. Patent No. 11,553,948, with other U.S. and international patents pending.
The OIC submitted a 510(k) to the FDA to demonstrate that the medical device is safe and effective, or “substantially equivalent,” to an existing FDA 510(k) cleared device. Upon 510(k) clearance by the FDA, the medical device may be legally marketed in the U.S.
Following this clearance, the OIC and the University of Utah PIVOT Center will establish industry partnerships to commercialize the Bone Bolt System and bring it to hospitals and surgery centers throughout the U.S.
“The simplicity of the Bone Bolt procedure to effectively stabilize complex fractures will impact the standard of care for patients with challenging fractures. The thoughtfulness during development of the Bone Bolt System encourages rapid adoption as the development team considered the care provider’s perspective in conjunction with clinical outcomes,” said PIVOT Associate Director of Innovation & Commercialization Huy Tran. “PIVOT Center is looking forward to engaging with potential industry partners to commercialize the technology and make an immediate impact on the quality of life for patients.”
Source: University of Utah Health

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







