
 Copy to clipboard
Copy to clipboard 
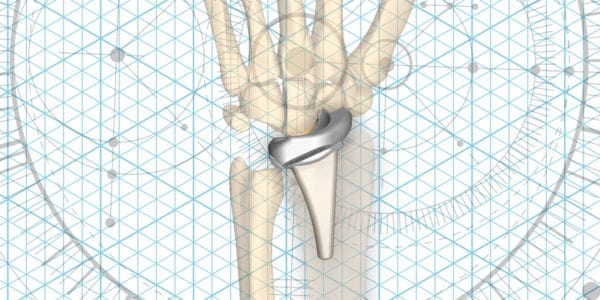
Extremity Medical received FDA 510(k) clearance for the KinematX Total Wrist, reportedly the first total wrist implant designed to emulate natural wrist range of motion in patients with wrist arthritis and other degenerative conditions. Launch will occur by the end of 2020.
The midcarpal implant is designed to negate common risks of radial implants such as limited range of motion, subluxation, distal component failure and osteolysis. The device is similar in design to the company’s KinematX Hemiarthroplasty Wrist that has been available ex-U.S. since 2011.
The design of KinematX was based on a new research approach to developing, identifying, tracking and measuring the precise motions of the eight bones in the human wrist. “The KinematX implant is scientifically designed to precisely replicate the complex natural articulations of the wrist that we documented in our research,” said Scott Wolfe, M.D., chief emeritus of Hand and Upper Extremity Service at Hospital for Special Surgery in New York and lead clinical designer of KinematX.
KinematX procedures will be performed on an outpatient basis. A patient registry will be established at Hospital for Special Surgery and other leading U.S. medical centers to track outcomes data.
Extremity Medical received FDA 510(k) clearance for the KinematX Total Wrist, reportedly the first total wrist implant designed to emulate natural wrist range of motion in patients with wrist arthritis and other degenerative conditions. Launch will occur by the end of 2020.
The midcarpal implant is designed to negate common risks of radial...
Extremity Medical received FDA 510(k) clearance for the KinematX Total Wrist, reportedly the first total wrist implant designed to emulate natural wrist range of motion in patients with wrist arthritis and other degenerative conditions. Launch will occur by the end of 2020.
The midcarpal implant is designed to negate common risks of radial implants such as limited range of motion, subluxation, distal component failure and osteolysis. The device is similar in design to the company’s KinematX Hemiarthroplasty Wrist that has been available ex-U.S. since 2011.
The design of KinematX was based on a new research approach to developing, identifying, tracking and measuring the precise motions of the eight bones in the human wrist. “The KinematX implant is scientifically designed to precisely replicate the complex natural articulations of the wrist that we documented in our research,” said Scott Wolfe, M.D., chief emeritus of Hand and Upper Extremity Service at Hospital for Special Surgery in New York and lead clinical designer of KinematX.
KinematX procedures will be performed on an outpatient basis. A patient registry will be established at Hospital for Special Surgery and other leading U.S. medical centers to track outcomes data.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







