
 Copy to clipboard
Copy to clipboard 
Expanding Innovations announced U.S. FDA 510(k) clearance for its N-GAGE Lumbar Plate System—the company’s first spinal fixation platform.
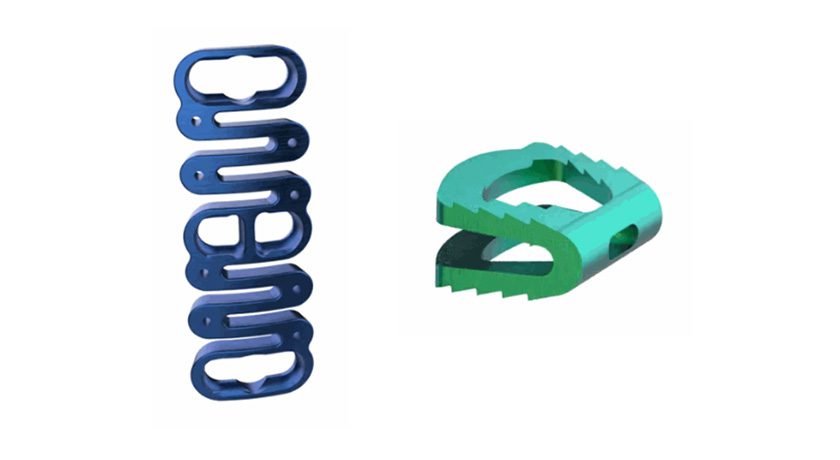
The modular, low-profile construct offers spine surgeons a flexible fixation solution for lateral and anterolateral lumbar fusions. The N-GAGE Plate pairs with the X-PAC LLIF Expandable Interbody Cage System via a NON-SCREW-based, Polyaxial Coupler. This first-of-its-kind connection simplifies plate-to-cage attachment and allows anatomical conformity to the vertebral bodies, mitigating the limitations of rigid, screw-based-attached lumbar plates.
“This regulatory milestone not only reflects our ongoing commitment to solving persistent challenges that exist in spine surgery,” said Ron Sacher, Executive Chairman and Co-Founder. “It represents the first of multiple upcoming technologies that will transition EI from a product-based technology company to a complete procedural solutions provider.”
Source: Expanding Innovations
Expanding Innovations announced U.S. FDA 510(k) clearance for its N-GAGE Lumbar Plate System—the company's first spinal fixation platform.
The modular, low-profile construct offers spine surgeons a flexible fixation solution for lateral and anterolateral lumbar fusions. The N-GAGE Plate pairs with the X-PAC LLIF Expandable Interbody Cage...
Expanding Innovations announced U.S. FDA 510(k) clearance for its N-GAGE Lumbar Plate System—the company’s first spinal fixation platform.
The modular, low-profile construct offers spine surgeons a flexible fixation solution for lateral and anterolateral lumbar fusions. The N-GAGE Plate pairs with the X-PAC LLIF Expandable Interbody Cage System via a NON-SCREW-based, Polyaxial Coupler. This first-of-its-kind connection simplifies plate-to-cage attachment and allows anatomical conformity to the vertebral bodies, mitigating the limitations of rigid, screw-based-attached lumbar plates.
“This regulatory milestone not only reflects our ongoing commitment to solving persistent challenges that exist in spine surgery,” said Ron Sacher, Executive Chairman and Co-Founder. “It represents the first of multiple upcoming technologies that will transition EI from a product-based technology company to a complete procedural solutions provider.”
Source: Expanding Innovations

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.