
 Copy to clipboard
Copy to clipboard 
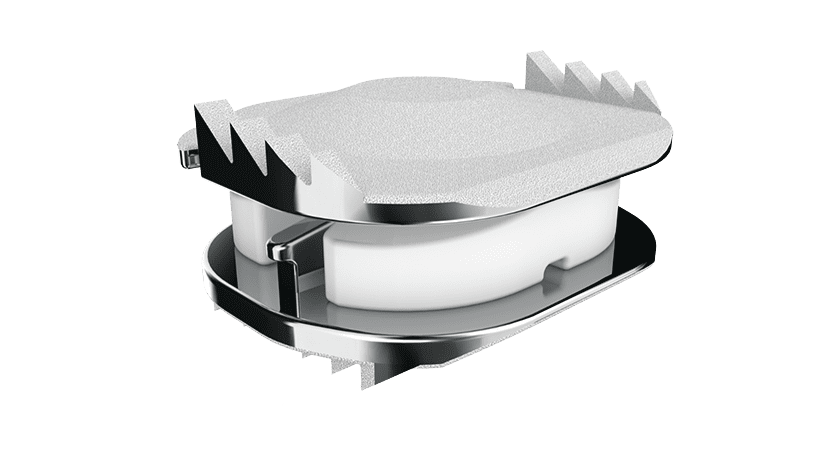
The French Republic published approval for the reimbursement of ZimVie’s Mobi-C Cervical Disc in both the public and private sectors in France. In addition, the clinical data for Mobi-C was awarded the highest quality rating of 10A* by the Orthopaedic Data Evaluation Panel (ODEP) in the United Kingdom. Physicians have used Mobi-C to treat patients in France and the U.K. since 2004.
In March, the French Republic’s Ministry of Health and Prevention issued a new version of the List of Products and Services that are reimbursable by health insurance and covered by the Health Insurance Fund (otherwise known as standard coverage). The list now includes the newly introduced medical device code 3174999 for “Cervical Disc Prostheses – Mobi-C Plug & Fit.” Implants included must be CE-marked devices that have a therapeutic, diagnostic or assistive added value, supported by clinical studies demonstrating the benefits of the solution.
Another validation of the Mobi-C Cervical Disc came through the recent 10A* rating from ODEP in the United Kingdom. ODEP provides the objective, systematic review and rating of the strength of evidence supporting the performance of medical devices. Its rating comprises several components. The numerical portion of the rating indicates the number of years of clinical evidence, with ten years representing full compliance with the benchmark from the National Institute for Health and Care Excellence. The “A” designates “Strong Evidence” based on a generally higher number of patients (giving greater confidence in the results presented), with all patients being subject to follow-up (their outcomes recorded). Finally, the addition of the star denotes a benchmark replacement rate of less than 1 in 20 (5%) at 10 years. There is no higher quality rating than A*.
“We applaud the French reimbursement decision for Mobi-C and welcome the exemplary 10A* rating from ODEP,” said Rebecca Whitney, Global President of ZimVie Spine. “We believe that these wins further validate the confidence that surgeons worldwide have demonstrated for Mobi-C since its first use in France in 2004 and FDA approval for one- and two-level use in the United States in 2013.”
Source: ZimVie
The French Republic published approval for the reimbursement of ZimVie's Mobi-C Cervical Disc in both the public and private sectors in France. In addition, the clinical data for Mobi-C was awarded the highest quality rating of 10A* by the Orthopaedic Data Evaluation Panel (ODEP) in the United Kingdom. Physicians have used Mobi-C to treat...
The French Republic published approval for the reimbursement of ZimVie’s Mobi-C Cervical Disc in both the public and private sectors in France. In addition, the clinical data for Mobi-C was awarded the highest quality rating of 10A* by the Orthopaedic Data Evaluation Panel (ODEP) in the United Kingdom. Physicians have used Mobi-C to treat patients in France and the U.K. since 2004.
In March, the French Republic’s Ministry of Health and Prevention issued a new version of the List of Products and Services that are reimbursable by health insurance and covered by the Health Insurance Fund (otherwise known as standard coverage). The list now includes the newly introduced medical device code 3174999 for “Cervical Disc Prostheses – Mobi-C Plug & Fit.” Implants included must be CE-marked devices that have a therapeutic, diagnostic or assistive added value, supported by clinical studies demonstrating the benefits of the solution.
Another validation of the Mobi-C Cervical Disc came through the recent 10A* rating from ODEP in the United Kingdom. ODEP provides the objective, systematic review and rating of the strength of evidence supporting the performance of medical devices. Its rating comprises several components. The numerical portion of the rating indicates the number of years of clinical evidence, with ten years representing full compliance with the benchmark from the National Institute for Health and Care Excellence. The “A” designates “Strong Evidence” based on a generally higher number of patients (giving greater confidence in the results presented), with all patients being subject to follow-up (their outcomes recorded). Finally, the addition of the star denotes a benchmark replacement rate of less than 1 in 20 (5%) at 10 years. There is no higher quality rating than A*.
“We applaud the French reimbursement decision for Mobi-C and welcome the exemplary 10A* rating from ODEP,” said Rebecca Whitney, Global President of ZimVie Spine. “We believe that these wins further validate the confidence that surgeons worldwide have demonstrated for Mobi-C since its first use in France in 2004 and FDA approval for one- and two-level use in the United States in 2013.”
Source: ZimVie

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







