
 Copy to clipboard
Copy to clipboard 
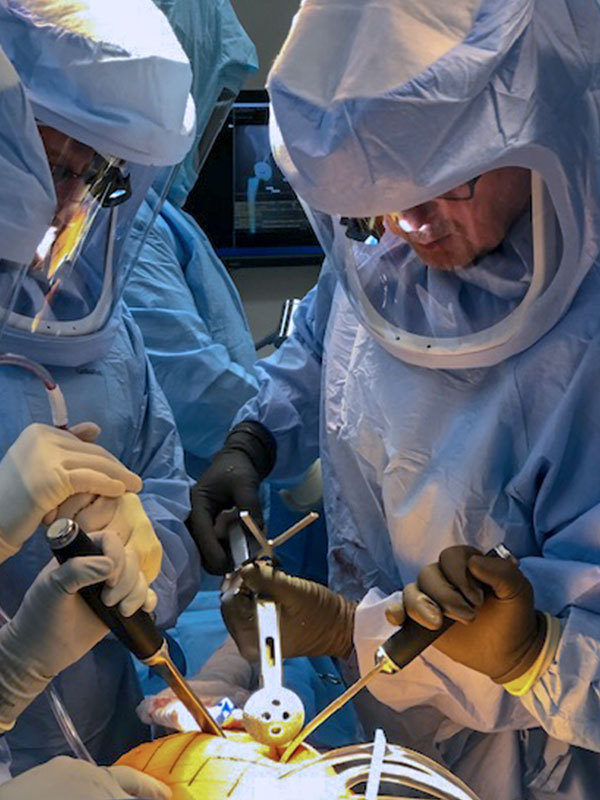
Exactech reported the successful first surgery using the Alteon® Acetabular Cup and XLE® Liner.
The device, which gained FDA 510(k) clearance earlier this month, provides multiple implant configurations and bearing options for the Alteon portfolio and features TAC™ asymmetric porous coating. XLE highly crosslinked vitamin-E enhanced acetabular liners undergo a patented gamma irradiation and mechanical annealing process, to achieve the desired crosslink density.
Exactech will commence U.S. rollout in 2H19.
Source: Exactech
Exactech reported the successful first surgery using the Alteon® Acetabular Cup and XLE® Liner.
The device, which gained FDA 510(k) clearance earlier this month, provides multiple implant configurations and bearing options for the Alteon portfolio and features TAC™ asymmetric porous coating. XLE highly crosslinked vitamin-E enhanced acetabular...
Exactech reported the successful first surgery using the Alteon® Acetabular Cup and XLE® Liner.
The device, which gained FDA 510(k) clearance earlier this month, provides multiple implant configurations and bearing options for the Alteon portfolio and features TAC™ asymmetric porous coating. XLE highly crosslinked vitamin-E enhanced acetabular liners undergo a patented gamma irradiation and mechanical annealing process, to achieve the desired crosslink density.
Exactech will commence U.S. rollout in 2H19.
Source: Exactech


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







