
 Copy to clipboard
Copy to clipboard 
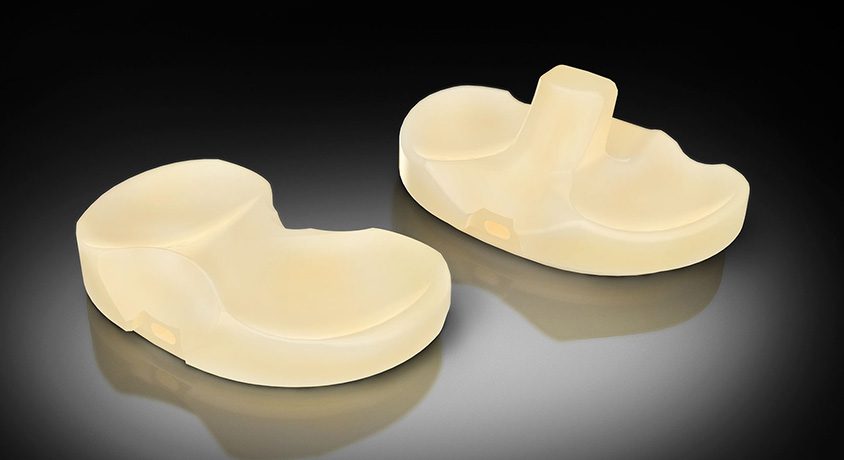
Exactech was granted FDA 510(k) clearance for its new, advanced Activit-E polyethylene for the Truliant knee replacement.
Activit-E brings a balance of material strength and toughness through chemically crosslinked polyethylene, while eliminating the need for gamma irradiation technology used in previous generations of polyethylene. The material is designed to maintain active oxidative resistance and long-term, high-performance, including strength and stabilization.
This new generation polyethylene is the latest, first-of-a-kind innovation by Orhun Muratoglu, Ph.D., Director of the Harris Orthopaedic Laboratory at Massachusetts General Hospital in Boston. Muratoglu invented the first crosslinked polyethylene, and the first of multiple generations of vitamin E, antioxidant polyethylene for leading orthopaedic companies.
The first products for Activit-E will launch in the beginning of 3Q23 for select U.S. customers. Global expansion will begin in 2024.
Source: Exactech
Exactech was granted FDA 510(k) clearance for its new, advanced Activit-E polyethylene for the Truliant knee replacement.
Activit-E brings a balance of material strength and toughness through chemically crosslinked polyethylene, while eliminating the need for gamma irradiation technology used in previous generations of polyethylene. The...
Exactech was granted FDA 510(k) clearance for its new, advanced Activit-E polyethylene for the Truliant knee replacement.
Activit-E brings a balance of material strength and toughness through chemically crosslinked polyethylene, while eliminating the need for gamma irradiation technology used in previous generations of polyethylene. The material is designed to maintain active oxidative resistance and long-term, high-performance, including strength and stabilization.
This new generation polyethylene is the latest, first-of-a-kind innovation by Orhun Muratoglu, Ph.D., Director of the Harris Orthopaedic Laboratory at Massachusetts General Hospital in Boston. Muratoglu invented the first crosslinked polyethylene, and the first of multiple generations of vitamin E, antioxidant polyethylene for leading orthopaedic companies.
The first products for Activit-E will launch in the beginning of 3Q23 for select U.S. customers. Global expansion will begin in 2024.
Source: Exactech

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







