
 Copy to clipboard
Copy to clipboard 
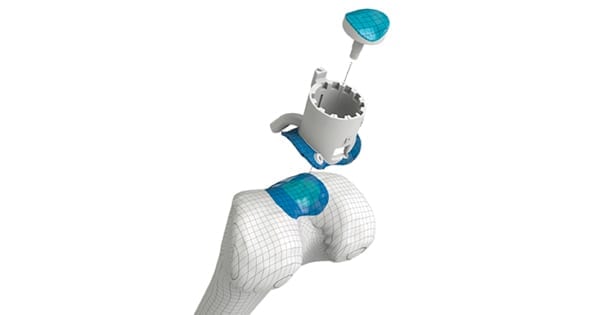
Episurf Medical announces the initiation of an investigator-initiated European multicentre study with 5 years’ follow-up of 50 Episealer® Knee patients. The study is a continuation of the European multicentre study, from which 2 years’ follow-up results were published in September 2020 .
The interim study from 2020 concluded that the results indicate that there is a definitive place for the Episealer® device in the management of a focal chondral or osteochondral defect affecting the distal femur. The study sponsor, investigator Associate Professor Tim Spalding, University Hospitals Coventry and Warwickshire NHS Trust, UK, will now perform the 5 years’ follow-up together with surgeons from 8 additional clinics in 6 European countries. Preliminary results from the follow-up are expected during the fourth quarter of 2021, while full results are expected in early 2022.
“This study can be performed thanks to the study group’s engagement and persistent follow-up of their patients, and we appreciate the initiative taken by Mr Tim Spalding. There has previously been a focus on 2 years’ data for the Episealer® patients. We are happy to now be in a position to get results from a large group of patients that have had their implants for 5 years. Recent publications with strong 2 years’ results have been very useful in Episurf’s dialog with healthcare providers, customers, and authorities in our markets. We are confident that the 5 years’ results will provide even stronger evidence in support of the Episealer®, showing that the technology is reliable over time” says Pål Ryfors, CEO Episurf Medical.
Source: Episurf Medical
Episurf Medical announces the initiation of an investigator-initiated European multicentre study with 5 years’ follow-up of 50 Episealer® Knee patients. The study is a continuation of the European multicentre study, from which 2 years’ follow-up results were published in September 2020 .
The interim study from 2020 concluded that the results...
Episurf Medical announces the initiation of an investigator-initiated European multicentre study with 5 years’ follow-up of 50 Episealer® Knee patients. The study is a continuation of the European multicentre study, from which 2 years’ follow-up results were published in September 2020 .
The interim study from 2020 concluded that the results indicate that there is a definitive place for the Episealer® device in the management of a focal chondral or osteochondral defect affecting the distal femur. The study sponsor, investigator Associate Professor Tim Spalding, University Hospitals Coventry and Warwickshire NHS Trust, UK, will now perform the 5 years’ follow-up together with surgeons from 8 additional clinics in 6 European countries. Preliminary results from the follow-up are expected during the fourth quarter of 2021, while full results are expected in early 2022.
“This study can be performed thanks to the study group’s engagement and persistent follow-up of their patients, and we appreciate the initiative taken by Mr Tim Spalding. There has previously been a focus on 2 years’ data for the Episealer® patients. We are happy to now be in a position to get results from a large group of patients that have had their implants for 5 years. Recent publications with strong 2 years’ results have been very useful in Episurf’s dialog with healthcare providers, customers, and authorities in our markets. We are confident that the 5 years’ results will provide even stronger evidence in support of the Episealer®, showing that the technology is reliable over time” says Pål Ryfors, CEO Episurf Medical.
Source: Episurf Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







