
 Copy to clipboard
Copy to clipboard 
Episurf Medical announced that results from a clinical study of the Episealer® Knee, with follow-up of the 30 first patients at nine Swedish sites, show good implant safety and significantly improved patient-reported outcome scores. The average follow-up time is 55 months (between 24-86 months).
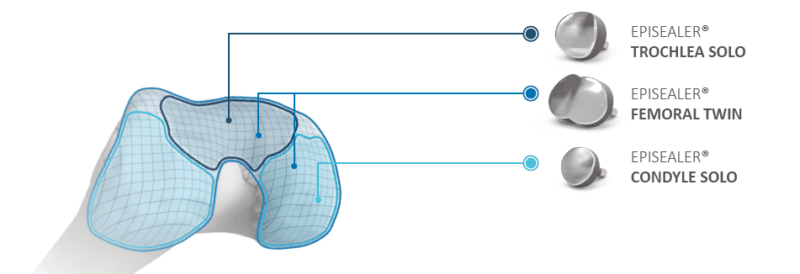
The Episealer Knee treats focal cartilage and osteochondral defects on the femoral side of the joint. The device is tailored to fit each patient’s curvature of the joint surface, size and shape of the lesion and underlying bone conditions. Individualized instrumentation enablies correct positioning, and associated imaging technology visualizes chondral and osteochondral defects.
The objective of the prospective consecutive cohort study is to assess subjective short-term outcome and possible longer-term risks of the intervention. Of the 30 patients assessed thus far, one has undergone revision to a partial knee.
“This is the third clinical study from which we see clinical results. To date, only one of them have been published, but as previously communicated, we are expecting plenty of activity from this field during 2020. We are very happy to hear about the positive results from this independent study. The results are in line with the strong data presented from an ongoing European multicentre study, and further, confirms the results we get reported from individual surgeons who follow their patients. It takes time to get long term results and we have now come to a time point when these anticipated results can be presented, and hopefully, soon published,” said Pål Ryfors, Episurf Medical CEO.
Episurf Medical announced that results from a clinical study of the Episealer® Knee, with follow-up of the 30 first patients at nine Swedish sites, show good implant safety and significantly improved patient-reported outcome scores. The average follow-up time is 55 months (between 24-86 months).
The Episealer Knee treats focal cartilage and...
Episurf Medical announced that results from a clinical study of the Episealer® Knee, with follow-up of the 30 first patients at nine Swedish sites, show good implant safety and significantly improved patient-reported outcome scores. The average follow-up time is 55 months (between 24-86 months).
The Episealer Knee treats focal cartilage and osteochondral defects on the femoral side of the joint. The device is tailored to fit each patient’s curvature of the joint surface, size and shape of the lesion and underlying bone conditions. Individualized instrumentation enablies correct positioning, and associated imaging technology visualizes chondral and osteochondral defects.
The objective of the prospective consecutive cohort study is to assess subjective short-term outcome and possible longer-term risks of the intervention. Of the 30 patients assessed thus far, one has undergone revision to a partial knee.
“This is the third clinical study from which we see clinical results. To date, only one of them have been published, but as previously communicated, we are expecting plenty of activity from this field during 2020. We are very happy to hear about the positive results from this independent study. The results are in line with the strong data presented from an ongoing European multicentre study, and further, confirms the results we get reported from individual surgeons who follow their patients. It takes time to get long term results and we have now come to a time point when these anticipated results can be presented, and hopefully, soon published,” said Pål Ryfors, Episurf Medical CEO.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







