
 Copy to clipboard
Copy to clipboard 
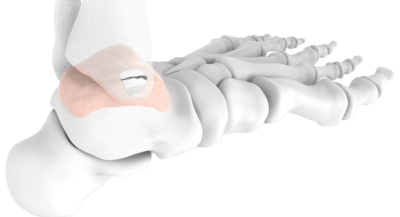
Episurf Medical received CE Mark approval for the Episealer® Talus and Talus Osteotomy Guide. The resurfacing implant is intended to treat osteochondral lesions of the talar dome in the ankle joint.
Talus implants are based on the same technology platform as the Episealer knee devices, with an individualized design based on medical imaging and 3D modulation.
Talus Osteotomy Guide is a patient-specific osteotomy guide to locate correct positioning and depth of the cut when performing a medial malleolar osteotomy to access the talar dome.
“We have been looking forward to this and we are enormously happy to finally have the CE Mark in place. It is fantastic that we now offer our implant technology not only for knees but also for these difficult to treat ankle injuries. Of course, this is also a significant commercial opportunity for us. We now have three commercial legs: knee joint, ankle and imaging,” said Pål Ryfors, CEO Episurf Medical.
“There is a great demand for this implant, as there are few alternatives for lesions in the ankle. In many cases, patients with these injuries are forced to undergo joint fusion. It feels fantastic to finally be able to offer our solution. This is an example that Episurf’s technology in principle can be applied to all joints,” said Leif Ryd, Senior Medical Advisor, Episurf Medical.
Episurf Medical received CE Mark approval for the Episealer® Talus and Talus Osteotomy Guide. The resurfacing implant is intended to treat osteochondral lesions of the talar dome in the ankle joint.
Talus implants are based on the same technology platform as the Episealer knee devices, with an individualized design based on medical imaging...
Episurf Medical received CE Mark approval for the Episealer® Talus and Talus Osteotomy Guide. The resurfacing implant is intended to treat osteochondral lesions of the talar dome in the ankle joint.
Talus implants are based on the same technology platform as the Episealer knee devices, with an individualized design based on medical imaging and 3D modulation.
Talus Osteotomy Guide is a patient-specific osteotomy guide to locate correct positioning and depth of the cut when performing a medial malleolar osteotomy to access the talar dome.
“We have been looking forward to this and we are enormously happy to finally have the CE Mark in place. It is fantastic that we now offer our implant technology not only for knees but also for these difficult to treat ankle injuries. Of course, this is also a significant commercial opportunity for us. We now have three commercial legs: knee joint, ankle and imaging,” said Pål Ryfors, CEO Episurf Medical.
“There is a great demand for this implant, as there are few alternatives for lesions in the ankle. In many cases, patients with these injuries are forced to undergo joint fusion. It feels fantastic to finally be able to offer our solution. This is an example that Episurf’s technology in principle can be applied to all joints,” said Leif Ryd, Senior Medical Advisor, Episurf Medical.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







