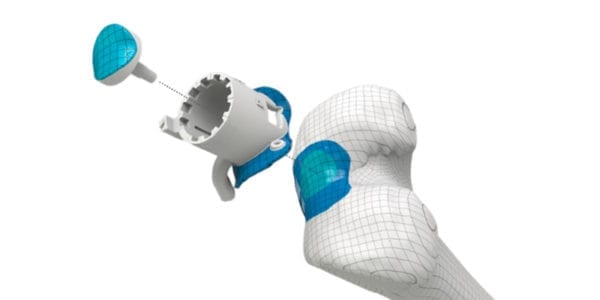






Episurf Medical’s system of pre-operative planning tools, individualized surgical instruments and an HA- and Ti-coated individualized Episealer® knee implants continue to deliver excellent results in multiple ongoing clinical trials. Updates include:
European multi-center study of the Episealer knee
- “Investigation of an Individualised Mini-metal Implant for Focal Cartilage Lesions in the Knee” follows 100 patients from nine clinics in six countries
- Study is fully enrolled and 70% of the patients have passed 24 months post-surgery
- Results show significant improvement and revision rate is at a very low level. These results were recently presented at ESSKA Speciality Days in Madrid.
Swedish multi-center study
- Study focusing on patient-reported outcome scores
- 22 patients have been subject to 2-year follow-up
Swedish multi-center study, longer term follow-up
- Ongoing study of five- to seven-year follow-up of first 10 first patients treated with Episealer; focus on patient-reported outcome scores
- Standing X-rays will be performed to investigate the progression of osteoarthritis
- Most of the patients have been examined, with final examinations to be performed shortly
Implant survival data
- Collecting data on device revision as part of postmarket surveillance
- Data will serve as a basis for cumulative revision rate analysis
Comparative joint kinematic study
- Comparative investigator-initiated study, “X-ray fluoroscopic analysis of knee joint kinematic in open and closed chain activities in patients with Episealer® Knee Implants”
- 10 patients that have undergone an Episealer procedure are followed-up and the joint mobility of the treated knee is assessed and compared with already available data from 10 healthy, non-treated knees, as well as with 10 knees that have undergone total knee replacement
- 80% of the Episealer patients have undergone examination and the remaining patients will be examined in early 2020
EPIC-Knee: Episealer Knee System IDE Clinical Study
- Prospective, randomized, controlled, multi-center study to evaluate the safety and effectiveness of Episealer vs. microfracture to treat focal knee chondral or osteochondral lesions in the U.S. and Europe
- 180 patients will be recruited with a 2:1 randomization of Episealer vs microfracture at eight sites in Germany, the U.K. and Denmark
“For several years, we have seen studies evaluating devices within the emerging orthopaedic segment of small implants being published. Based on the results from the European multi-center Episealer study, we are convinced that ongoing studies will show the strength and utility of our technology. If we can demonstrate strong clinical results, combined with fast rehab and high implant survival rates, we have met our original hypothesis that the combination of a pre-operative planning tool, individualized surgical instruments and an HA- and Ti-coated individualized implant delivers excellent results. This clinical pipeline is very interesting, with several important milestones in the near future,” said Pål Ryfors, Episurf CEO.
Episurf Medical's system of pre-operative planning tools, individualized surgical instruments and an HA- and Ti-coated individualized Episealer® knee implants continue to deliver excellent results in multiple ongoing clinical trials. Updates include:
European multi-center study of the Episealer knee
“Investigation of an Individualised...
Episurf Medical’s system of pre-operative planning tools, individualized surgical instruments and an HA- and Ti-coated individualized Episealer® knee implants continue to deliver excellent results in multiple ongoing clinical trials. Updates include:
European multi-center study of the Episealer knee
- “Investigation of an Individualised Mini-metal Implant for Focal Cartilage Lesions in the Knee” follows 100 patients from nine clinics in six countries
- Study is fully enrolled and 70% of the patients have passed 24 months post-surgery
- Results show significant improvement and revision rate is at a very low level. These results were recently presented at ESSKA Speciality Days in Madrid.
Swedish multi-center study
- Study focusing on patient-reported outcome scores
- 22 patients have been subject to 2-year follow-up
Swedish multi-center study, longer term follow-up
- Ongoing study of five- to seven-year follow-up of first 10 first patients treated with Episealer; focus on patient-reported outcome scores
- Standing X-rays will be performed to investigate the progression of osteoarthritis
- Most of the patients have been examined, with final examinations to be performed shortly
Implant survival data
- Collecting data on device revision as part of postmarket surveillance
- Data will serve as a basis for cumulative revision rate analysis
Comparative joint kinematic study
- Comparative investigator-initiated study, “X-ray fluoroscopic analysis of knee joint kinematic in open and closed chain activities in patients with Episealer® Knee Implants”
- 10 patients that have undergone an Episealer procedure are followed-up and the joint mobility of the treated knee is assessed and compared with already available data from 10 healthy, non-treated knees, as well as with 10 knees that have undergone total knee replacement
- 80% of the Episealer patients have undergone examination and the remaining patients will be examined in early 2020
EPIC-Knee: Episealer Knee System IDE Clinical Study
- Prospective, randomized, controlled, multi-center study to evaluate the safety and effectiveness of Episealer vs. microfracture to treat focal knee chondral or osteochondral lesions in the U.S. and Europe
- 180 patients will be recruited with a 2:1 randomization of Episealer vs microfracture at eight sites in Germany, the U.K. and Denmark
“For several years, we have seen studies evaluating devices within the emerging orthopaedic segment of small implants being published. Based on the results from the European multi-center Episealer study, we are convinced that ongoing studies will show the strength and utility of our technology. If we can demonstrate strong clinical results, combined with fast rehab and high implant survival rates, we have met our original hypothesis that the combination of a pre-operative planning tool, individualized surgical instruments and an HA- and Ti-coated individualized implant delivers excellent results. This clinical pipeline is very interesting, with several important milestones in the near future,” said Pål Ryfors, Episurf CEO.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







