
 Copy to clipboard
Copy to clipboard 
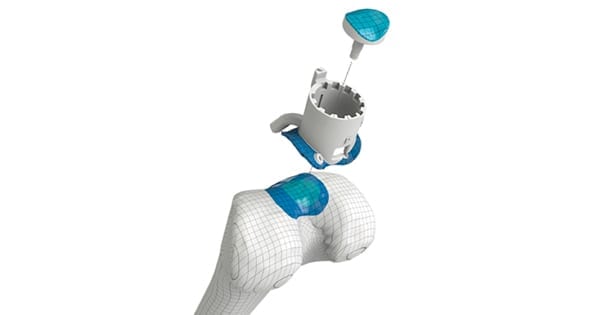
Episurf Medical announced the first surgery in the company’s Investigational Device Exemption (IDE) clinical trial, EPIC-Knee: Episealer® Knee System IDE Clinical Study, in Europe. The first IDE study surgery in the U.S. is slated for later this month.
Further, the company received positive results in a clinical study with follow-up at five to seven years with the 10 first patients to receive an Episealer Knee in a multicenter Sweden study.
Patients in the study received an Episealer Knee implant as treatment of a focal cartilage defect in the knee from 2012 to 2014. Studies indicate that positive results at 24 months’ follow-up, previously presented, remain unchanged at an average follow-up time of 75 months, with no revisions.
“This is the first clinical study with 5-7 years’ follow up from the use of the Episealer® knee implants. The fact that the positive results that are shown at the 24 months’ follow-up remain over time, points to the conclusion that our technology is not only a temporary solution. Stakeholders, including our customers, insurance companies and healthcare providers, have requested this type of clinical evidence before approving the Episealer® technology within their standard treatment algorithm. We are delighted to hear about the positive results from this independent study, and we hope to see these results published shortly,” said Pål Ryfors, CEO.
Episurf Medical announced the first surgery in the company's Investigational Device Exemption (IDE) clinical trial, EPIC-Knee: Episealer® Knee System IDE Clinical Study, in Europe. The first IDE study surgery in the U.S. is slated for later this month.
Further, the company received positive results in a clinical study with follow-up at...
Episurf Medical announced the first surgery in the company’s Investigational Device Exemption (IDE) clinical trial, EPIC-Knee: Episealer® Knee System IDE Clinical Study, in Europe. The first IDE study surgery in the U.S. is slated for later this month.
Further, the company received positive results in a clinical study with follow-up at five to seven years with the 10 first patients to receive an Episealer Knee in a multicenter Sweden study.
Patients in the study received an Episealer Knee implant as treatment of a focal cartilage defect in the knee from 2012 to 2014. Studies indicate that positive results at 24 months’ follow-up, previously presented, remain unchanged at an average follow-up time of 75 months, with no revisions.
“This is the first clinical study with 5-7 years’ follow up from the use of the Episealer® knee implants. The fact that the positive results that are shown at the 24 months’ follow-up remain over time, points to the conclusion that our technology is not only a temporary solution. Stakeholders, including our customers, insurance companies and healthcare providers, have requested this type of clinical evidence before approving the Episealer® technology within their standard treatment algorithm. We are delighted to hear about the positive results from this independent study, and we hope to see these results published shortly,” said Pål Ryfors, CEO.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







