
 Copy to clipboard
Copy to clipboard 
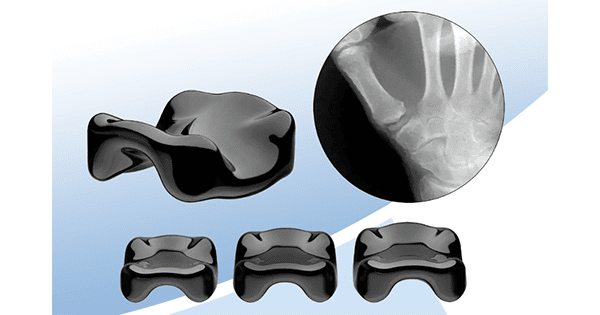
Ensemble Orthopedics received FDA 510(k) clearance to market the Ensemble CMC Implant to treat early-stage osteoarthritis of the carpometacarpal (CMC) joint in a minimally invasive procedure.
Ensemble CMC is based on On-X® PyroCarbon, a proprietary form of pyrolytic carbon produced by On-X Life Technologies. The high strength, low modulus material has shown to offer superior wear resistance when bearing against bone than metal or ceramic.
Ensemble CMC has a proprietary saddle shape that replaces the natural bearing surfaces of the carpal and metacarpal bones with an interpositional implant. This combines minimal joint resection with a stable design suitable for higher demand patients.
Ensemble CMC is inserted with minimal disruption to the joint capsule and preservation of critical stabilizing soft tissues offering the potential for faster rehabilitation.
Ensemble Orthopedics received FDA 510(k) clearance to market the Ensemble CMC Implant to treat early-stage osteoarthritis of the carpometacarpal (CMC) joint in a minimally invasive procedure.
Ensemble CMC is based on On-X® PyroCarbon, a proprietary form of pyrolytic carbon produced by On-X Life Technologies. The high strength, low modulus...
Ensemble Orthopedics received FDA 510(k) clearance to market the Ensemble CMC Implant to treat early-stage osteoarthritis of the carpometacarpal (CMC) joint in a minimally invasive procedure.
Ensemble CMC is based on On-X® PyroCarbon, a proprietary form of pyrolytic carbon produced by On-X Life Technologies. The high strength, low modulus material has shown to offer superior wear resistance when bearing against bone than metal or ceramic.
Ensemble CMC has a proprietary saddle shape that replaces the natural bearing surfaces of the carpal and metacarpal bones with an interpositional implant. This combines minimal joint resection with a stable design suitable for higher demand patients.
Ensemble CMC is inserted with minimal disruption to the joint capsule and preservation of critical stabilizing soft tissues offering the potential for faster rehabilitation.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







