
 Copy to clipboard
Copy to clipboard 
Ensemble Orthopedics marked the 700th implantation of the Ensemble CMC, an increase from 100 cases two years ago. This latest milestone coincides with the third anniversary of the device’s first implantation.
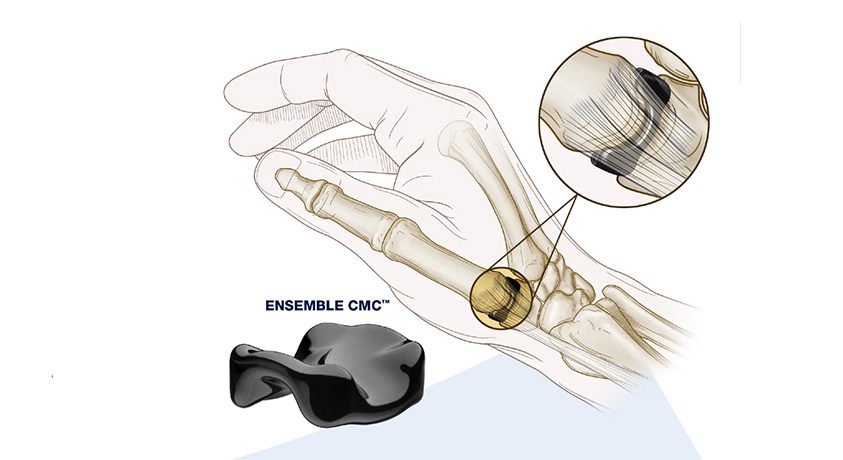
The Ensemble CMC joint replacement was developed as a minimally invasive treatment of carpometacarpal (CMC, or thumb) osteoarthritis, and received FDA 510(k) marketing clearance in late 2020.
Since the launch of the device, Ensemble Orthopedics has enjoyed a doubling of company sales year-over-year for the last three years. Beyond the CMC, Ensemble is dedicated to bringing this stemless, interpositional philosophy to other joints of the hand and wrist.
Ensemble CMC was designed to treat a broader range of patients using a minimally invasive procedure that offers potential for shorter recovery times as well as improvement in pain and joint function. The implant’s saddle shape maintains patient anatomy and, in contrast to a traditional ligament reconstruction and tendon interposition (LRTI) procedure, the Ensemble CMC preserves the trapezium by replacing only the damaged bearing surfaces of the trapezium and metacarpal bones.
Ensemble CMC is constructed using On-X Carbon (pyrocarbon), a high-strength, low-friction, biocompatible material which has shown superior wear resistance against bone when compared to metals or ceramic.
Source: Ensemble Orthopedics, Inc.
Ensemble Orthopedics marked the 700th implantation of the Ensemble CMC, an increase from 100 cases two years ago. This latest milestone coincides with the third anniversary of the device’s first implantation.
The Ensemble CMC joint replacement was developed as a minimally invasive treatment of carpometacarpal (CMC, or thumb)...
Ensemble Orthopedics marked the 700th implantation of the Ensemble CMC, an increase from 100 cases two years ago. This latest milestone coincides with the third anniversary of the device’s first implantation.
The Ensemble CMC joint replacement was developed as a minimally invasive treatment of carpometacarpal (CMC, or thumb) osteoarthritis, and received FDA 510(k) marketing clearance in late 2020.
Since the launch of the device, Ensemble Orthopedics has enjoyed a doubling of company sales year-over-year for the last three years. Beyond the CMC, Ensemble is dedicated to bringing this stemless, interpositional philosophy to other joints of the hand and wrist.
Ensemble CMC was designed to treat a broader range of patients using a minimally invasive procedure that offers potential for shorter recovery times as well as improvement in pain and joint function. The implant’s saddle shape maintains patient anatomy and, in contrast to a traditional ligament reconstruction and tendon interposition (LRTI) procedure, the Ensemble CMC preserves the trapezium by replacing only the damaged bearing surfaces of the trapezium and metacarpal bones.
Ensemble CMC is constructed using On-X Carbon (pyrocarbon), a high-strength, low-friction, biocompatible material which has shown superior wear resistance against bone when compared to metals or ceramic.
Source: Ensemble Orthopedics, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







