
 Copy to clipboard
Copy to clipboard 
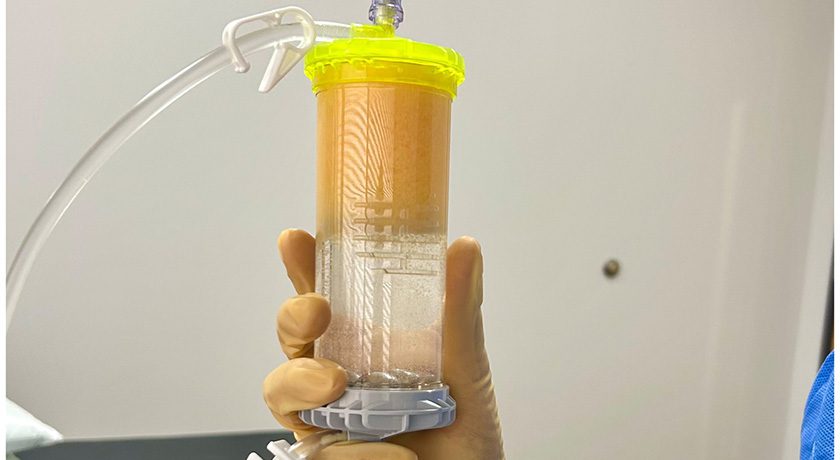
Lipogems enrolled the final patient in the ARISE II U.S. FDA IDE Study, which aims to evaluate the efficacy and safety of MicroFat injections compared to saline for the treatment of knee osteoarthritis (OA). The double-blinded, randomized, controlled trial enrolled 173 subjects in nine months across 16 U.S. sites. The primary endpoints evaluate improvement in pain and function at six months post-injection. Efficacy and safety results from ARISE II are expected to be announced in late 2025.
The ARISE I and II studies are designed to support Lipogems in obtaining a separate indication specifically for knee OA. While the Lipogems device has been FDA cleared for use in orthopedics and arthroscopic surgery for the closed-loop processing and transfer of lipoaspirate tissue for 10 years, these studies represent the largest clinical trials to date for the company.
“As a responsible company, the goal of these studies is to prove the efficacy of Lipogems for this patient population and ultimately apply and obtain reimbursement for this indication making the technology more available for the growing demand of patients that have tried conservative options with limited relief and are not ready for a knee replacement. Lipogems is excited to have received confirmation from FDA to apply for a modular Premarket Approval Application and has already moved forward with that process,” said Carl Llewellyn, Chief Executive Officer, Lipogems USA.
Source: Lipogems
Lipogems enrolled the final patient in the ARISE II U.S. FDA IDE Study, which aims to evaluate the efficacy and safety of MicroFat injections compared to saline for the treatment of knee osteoarthritis (OA). The double-blinded, randomized, controlled trial enrolled 173 subjects in nine months across 16 U.S. sites. The primary endpoints evaluate...
Lipogems enrolled the final patient in the ARISE II U.S. FDA IDE Study, which aims to evaluate the efficacy and safety of MicroFat injections compared to saline for the treatment of knee osteoarthritis (OA). The double-blinded, randomized, controlled trial enrolled 173 subjects in nine months across 16 U.S. sites. The primary endpoints evaluate improvement in pain and function at six months post-injection. Efficacy and safety results from ARISE II are expected to be announced in late 2025.
The ARISE I and II studies are designed to support Lipogems in obtaining a separate indication specifically for knee OA. While the Lipogems device has been FDA cleared for use in orthopedics and arthroscopic surgery for the closed-loop processing and transfer of lipoaspirate tissue for 10 years, these studies represent the largest clinical trials to date for the company.
“As a responsible company, the goal of these studies is to prove the efficacy of Lipogems for this patient population and ultimately apply and obtain reimbursement for this indication making the technology more available for the growing demand of patients that have tried conservative options with limited relief and are not ready for a knee replacement. Lipogems is excited to have received confirmation from FDA to apply for a modular Premarket Approval Application and has already moved forward with that process,” said Carl Llewellyn, Chief Executive Officer, Lipogems USA.
Source: Lipogems

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







