
 Copy to clipboard
Copy to clipboard 
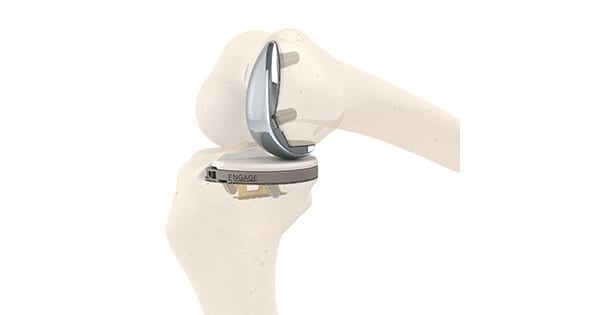
Engage Surgical gained FDA 510(k) clearance to market the only cementless partial knee system available in the U.S. The Engage™ Partial Knee is cleared for both cementless or cemented fixation, and will be available initially to surgeons at Hospital for Special Surgery and affiliated ambulatory surgical centers, and to select surgeons at participating facilities throughout the U.S.
Engage Partial offers porous implants for promoting biological fixation on both the tibia and femur. A 3D-printed tibial tray features Engage Anchor technology for immediate stability at the tibial interface and the titanium Affinium3D™ ultra-porous bone ingrowth surface that promotes long-term cementless biological fixation.
Engage Partial is implanted by a reproducible, ligament-guided surgical technique inspired by robotic methods, but using precise manual instrumentation to avoid added setup time and extra expense associated with robotic and navigation techniques.
Dan Justin, CEO and Co-Founder, said, “Before founding our company, we asked knee surgeons what problems we could help solve. Repeatedly, surgeons mentioned that active patients are more satisfied with their knee function when healthy articular cartilage and ligament balance is preserved. A stable, non-cemented, ligament preserving, fixed bearing partial knee system was needed. To solve this problem, we brought together an experienced team of surgeons, engineers, and manufacturers who are proud to introduce this groundbreaking system.”
Engage Surgical gained FDA 510(k) clearance to market the only cementless partial knee system available in the U.S. The Engage™ Partial Knee is cleared for both cementless or cemented fixation, and will be available initially to surgeons at Hospital for Special Surgery and affiliated ambulatory surgical centers, and to select surgeons at...
Engage Surgical gained FDA 510(k) clearance to market the only cementless partial knee system available in the U.S. The Engage™ Partial Knee is cleared for both cementless or cemented fixation, and will be available initially to surgeons at Hospital for Special Surgery and affiliated ambulatory surgical centers, and to select surgeons at participating facilities throughout the U.S.
Engage Partial offers porous implants for promoting biological fixation on both the tibia and femur. A 3D-printed tibial tray features Engage Anchor technology for immediate stability at the tibial interface and the titanium Affinium3D™ ultra-porous bone ingrowth surface that promotes long-term cementless biological fixation.
Engage Partial is implanted by a reproducible, ligament-guided surgical technique inspired by robotic methods, but using precise manual instrumentation to avoid added setup time and extra expense associated with robotic and navigation techniques.
Dan Justin, CEO and Co-Founder, said, “Before founding our company, we asked knee surgeons what problems we could help solve. Repeatedly, surgeons mentioned that active patients are more satisfied with their knee function when healthy articular cartilage and ligament balance is preserved. A stable, non-cemented, ligament preserving, fixed bearing partial knee system was needed. To solve this problem, we brought together an experienced team of surgeons, engineers, and manufacturers who are proud to introduce this groundbreaking system.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







