
 Copy to clipboard
Copy to clipboard 
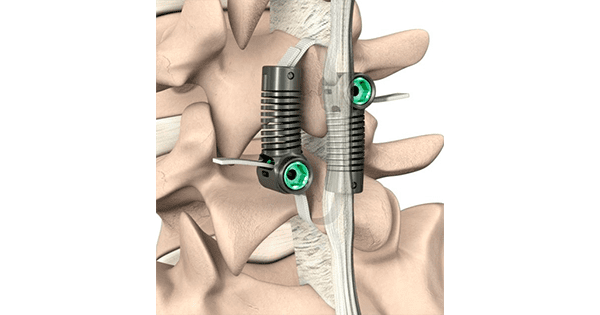
Empirical Spine submitted its Premarket Approval (PMA) Module II to FDA for its LimiFlex™ Dynamic Sagittal Tether™ (DST).
DST is intended to be an alternative to spinal fusion. LimiFlex works with the body’s anatomy to provide the incremental stability needed by maintaining lordosis and encouraging facet engagement. Unlike interspinous spacers, LimiFlex only engages when a patient bends forward, which avoids delivering excess force and stress on the spinous processes with each step.
LimiFlex was designed to act as a ligament to augment the natural anatomy after a thorough decompression for the stenosis. It is compatible with current decompression techniques and is inserted without screws to reduce procedural morbidity and preserve the option for other treatments if needed.
LimiFlex received the CE Mark in 2009 and has been implanted with excellent results in more than 2,000 European patients. Results to date show the potential for LimiFlex to provide a robust, motion-preserving, minimally invasive outpatient solution. The company was granted FDA Breakthrough Device Designation for the LimiFlex Paraspinous Tension Band one year ago.
Source: LimiFlex
Empirical Spine submitted its Premarket Approval (PMA) Module II to FDA for its LimiFlex™ Dynamic Sagittal Tether™ (DST).
DST is intended to be an alternative to spinal fusion. LimiFlex works with the body's anatomy to provide the incremental stability needed by maintaining lordosis and encouraging facet engagement. Unlike interspinous...
Empirical Spine submitted its Premarket Approval (PMA) Module II to FDA for its LimiFlex™ Dynamic Sagittal Tether™ (DST).
DST is intended to be an alternative to spinal fusion. LimiFlex works with the body’s anatomy to provide the incremental stability needed by maintaining lordosis and encouraging facet engagement. Unlike interspinous spacers, LimiFlex only engages when a patient bends forward, which avoids delivering excess force and stress on the spinous processes with each step.
LimiFlex was designed to act as a ligament to augment the natural anatomy after a thorough decompression for the stenosis. It is compatible with current decompression techniques and is inserted without screws to reduce procedural morbidity and preserve the option for other treatments if needed.
LimiFlex received the CE Mark in 2009 and has been implanted with excellent results in more than 2,000 European patients. Results to date show the potential for LimiFlex to provide a robust, motion-preserving, minimally invasive outpatient solution. The company was granted FDA Breakthrough Device Designation for the LimiFlex Paraspinous Tension Band one year ago.
Source: LimiFlex

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







