
 Copy to clipboard
Copy to clipboard 
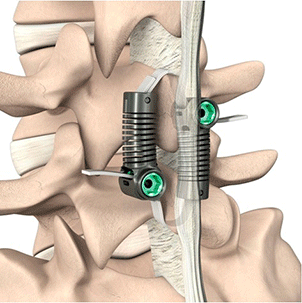
Empirical Spine LimiFlex
Paraspinous Tension Band
Empirical Spine completed enrollment in the investigational arm of its U.S. Investigational Device Exemption trial of the LimiFlex Paraspinous Tension Band with decompression to treat degenerative spondylolisthesis with lumbar spinal stenosis.
The trial compares outcomes of patients who receive either LimiFlex or transforaminal lumbar interbody fusion following a decompression procedure. The target of 135 LimiFlex patients are enrolled in the investigational arm. The control arm will continue enrolling toward its target of 160 patients. Trial results will support a premarketing approval application to the FDA.
The LimiFlex was developed as a motion-preserving alternative to the use of instrumentation and bone graft to stabilize the spine, post-decompression. The prospective, multi-center, controlled clinical trial will evaluate the safety and efficacy of the device compared to fusion, with a goal of showing that patients can achieve fusion-equivalent results, without the fusion.
The device received approval under the CE Mark in 2009 and has been implanted in over 2,000 European patients.
Empirical Spine completed enrollment in the investigational arm of its U.S. Investigational Device Exemption trial of the LimiFlex Paraspinous Tension Band with decompression to treat degenerative spondylolisthesis with lumbar spinal stenosis.
The trial compares outcomes of patients who receive either LimiFlex or transforaminal lumbar...

Empirical Spine LimiFlex
Paraspinous Tension Band
Empirical Spine completed enrollment in the investigational arm of its U.S. Investigational Device Exemption trial of the LimiFlex Paraspinous Tension Band with decompression to treat degenerative spondylolisthesis with lumbar spinal stenosis.
The trial compares outcomes of patients who receive either LimiFlex or transforaminal lumbar interbody fusion following a decompression procedure. The target of 135 LimiFlex patients are enrolled in the investigational arm. The control arm will continue enrolling toward its target of 160 patients. Trial results will support a premarketing approval application to the FDA.
The LimiFlex was developed as a motion-preserving alternative to the use of instrumentation and bone graft to stabilize the spine, post-decompression. The prospective, multi-center, controlled clinical trial will evaluate the safety and efficacy of the device compared to fusion, with a goal of showing that patients can achieve fusion-equivalent results, without the fusion.
The device received approval under the CE Mark in 2009 and has been implanted in over 2,000 European patients.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







