
 Copy to clipboard
Copy to clipboard 
Empirical Spine has completed the final step in the FDA submission process for the LimiFlex™ Dynamic Sagittal Tether™ (DST). The Premarket Approval (PMA) submission included Module III, with data and analysis of the two-year results from the pivotal Investigational Device Exemption (IDE) clinical trial comparing LimiFlex DST stabilization versus fusion surgery for degenerative spondylolisthesis patients with spinal stenosis.
LimiFlex DST is a minimally invasive, outpatient surgical option for this specific patient population. The device previously received FDA’s Breakthrough Designation for its potential to provide advantages over currently approved spinal stabilization technologies.
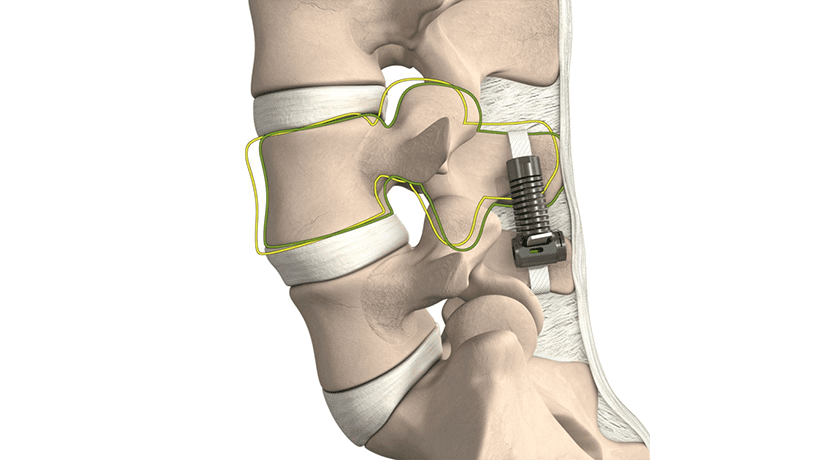
The LimiFlex DST uses a different concept than past devices to provide durable symptom resolution while preserving spinal motion. LimiFlex’s design creates an elastic resistance to flexion and maintains lordosis, addressing the root problem of excessive instability at the index segment after decompression surgery. It is reportedly the only device designed to restore the natural flexion stability of the lumbar spine by mimicking and augmenting the anatomic ligaments to create natural motion across the spine without invasive screws, rods, and bone grafts. After completion of the neural decompression surgery, LimiFlex DST is implanted through the same incision to restore stability while maintaining balanced mobility of the spine.
The PMA Module III submission provided data from the prospective, multi-center, controlled IDE trial comparing LimiFlex to interbody fusion, considered the standard of care. The primary outcome is the two-year results of a composite endpoint comprising functional, neurologic, redo surgery and device integrity components. The study, which enrolled 299 patients across 27 U.S. spine centers, will continue to follow trial participants out to five years.
Source: Empirical Spine
Empirical Spine has completed the final step in the FDA submission process for the LimiFlex™ Dynamic Sagittal Tether™ (DST). The Premarket Approval (PMA) submission included Module III, with data and analysis of the two-year results from the pivotal Investigational Device Exemption (IDE) clinical trial comparing LimiFlex DST stabilization versus...
Empirical Spine has completed the final step in the FDA submission process for the LimiFlex™ Dynamic Sagittal Tether™ (DST). The Premarket Approval (PMA) submission included Module III, with data and analysis of the two-year results from the pivotal Investigational Device Exemption (IDE) clinical trial comparing LimiFlex DST stabilization versus fusion surgery for degenerative spondylolisthesis patients with spinal stenosis.
LimiFlex DST is a minimally invasive, outpatient surgical option for this specific patient population. The device previously received FDA’s Breakthrough Designation for its potential to provide advantages over currently approved spinal stabilization technologies.
The LimiFlex DST uses a different concept than past devices to provide durable symptom resolution while preserving spinal motion. LimiFlex’s design creates an elastic resistance to flexion and maintains lordosis, addressing the root problem of excessive instability at the index segment after decompression surgery. It is reportedly the only device designed to restore the natural flexion stability of the lumbar spine by mimicking and augmenting the anatomic ligaments to create natural motion across the spine without invasive screws, rods, and bone grafts. After completion of the neural decompression surgery, LimiFlex DST is implanted through the same incision to restore stability while maintaining balanced mobility of the spine.
The PMA Module III submission provided data from the prospective, multi-center, controlled IDE trial comparing LimiFlex to interbody fusion, considered the standard of care. The primary outcome is the two-year results of a composite endpoint comprising functional, neurologic, redo surgery and device integrity components. The study, which enrolled 299 patients across 27 U.S. spine centers, will continue to follow trial participants out to five years.
Source: Empirical Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







