
 Copy to clipboard
Copy to clipboard 
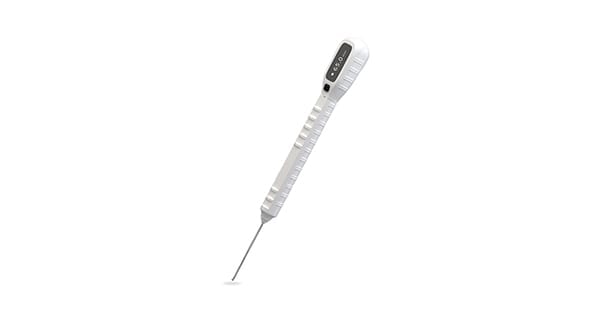
EDGe Surgical received approval under the CE Mark for the EDG® Ortho 65mm single-use electronic depth gauge.
The EDG Ortho is a sterile, single-use device used to more accurately and safely measure which length screw should be implanted into a patient during orthopedic fracture surgeries.
Christopher Wilson, CEO, EDGe Surgical, said, “Preparing for the requirements under the new MDR is placing significant resource strain on large orthopedic manufacturers, and some have made the tough decision to discontinue certain products due to lack of readiness. Our EDG Ortho electronic depth gauge is a readily available option for manufacturers that are having difficulty transitioning their legacy metal depth gauges to the more stringent requirements.”
EDGe Surgical received approval under the CE Mark for the EDG® Ortho 65mm single-use electronic depth gauge.
The EDG Ortho is a sterile, single-use device used to more accurately and safely measure which length screw should be implanted into a patient during orthopedic fracture surgeries.
Christopher Wilson, CEO, EDGe Surgical, said,...
EDGe Surgical received approval under the CE Mark for the EDG® Ortho 65mm single-use electronic depth gauge.
The EDG Ortho is a sterile, single-use device used to more accurately and safely measure which length screw should be implanted into a patient during orthopedic fracture surgeries.
Christopher Wilson, CEO, EDGe Surgical, said, “Preparing for the requirements under the new MDR is placing significant resource strain on large orthopedic manufacturers, and some have made the tough decision to discontinue certain products due to lack of readiness. Our EDG Ortho electronic depth gauge is a readily available option for manufacturers that are having difficulty transitioning their legacy metal depth gauges to the more stringent requirements.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







