Copy to clipboard
Copy to clipboard 
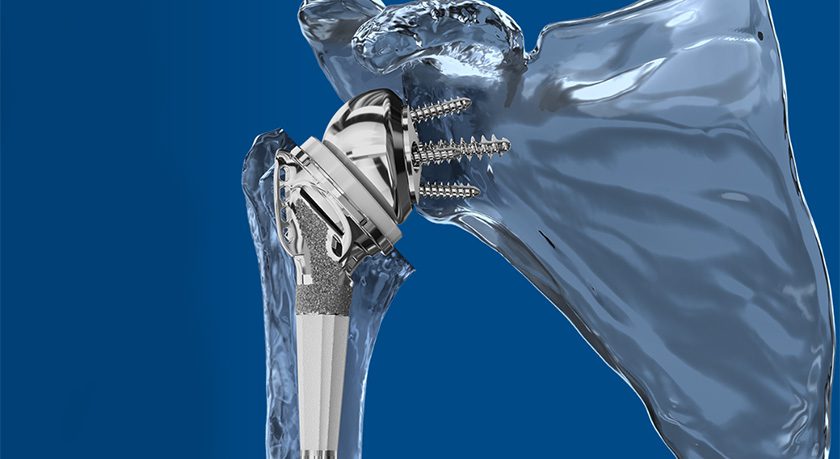
EDGe Surgical closed a $1.4MM Seed Round to fund launch of its Ortho EDG®, a single-use electronic depth gauge that assists surgeons in placing orthopaedic screws.
The FDA 510(k) cleared device is designed to address precision limitations and infection risks associated with the standard depth gauge.
Ortho EDG® is EDGe Surgical’s first device. The company, founded in 2015, recently opened a $2MM Series A to develop Spine EDG® for the spine market.
Source: EDGe Surgical
EDGe Surgical closed a $1.4MM Seed Round to fund launch of its Ortho EDG®, a single-use electronic depth gauge that assists surgeons in placing orthopaedic screws.
The FDA 510(k) cleared device is designed to address precision limitations...
EDGe Surgical closed a $1.4MM Seed Round to fund launch of its Ortho EDG®, a single-use electronic depth gauge that assists surgeons in placing orthopaedic screws.
The FDA 510(k) cleared device is designed to address precision limitations and infection risks associated with the standard depth gauge.
Ortho EDG® is EDGe Surgical’s first device. The company, founded in 2015, recently opened a $2MM Series A to develop Spine EDG® for the spine market.
Source: EDGe Surgical

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.