
 Copy to clipboard
Copy to clipboard 
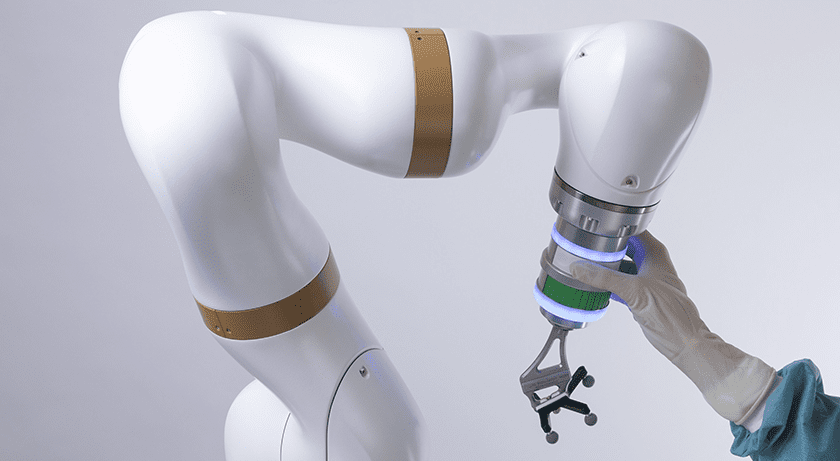
eCential Robotics was granted FDA 510(k) clearance for its 3D imaging, navigation and robotics guidance system.
eCential Robotics previously obtained CE Mark approval and launched its first platform in France. Ten units have been sold and installed in Europe, with more than two thousand surgeries performed. FDA 510(k) clearance of the intra-operative 2D and 3D imaging, navigation and robotic guidance platform, with a first universal application in spine surgery, now secures the plan to access the North American market over the next few years.
The platform unifies intraoperative 2D/3D imaging, navigation and robotics and avoids the pitfalls of traditional image-navigation pairing, such as unreliable calibration and registration steps, and streamlines the surgical workflow by automating numerous technical steps. A single user interface for all devices enables to control imaging, navigation and robotic functionalities from a single input and output graphical display screen.
The system is also a fully open solution, meaning that it can be used with any manufacturer’s implants. Built around a range of applications currently dedicated to spine surgery, the eCential Robotics platform will in the future expand to multiple bone surgery indications.
The unified open platform provides 2D/3D medical imaging and stereotaxic guidance. The system consists of three mobile interconnected units: a mobile C-arm, a mobile viewing workstation and a mobile collaborative robot. The mobile X-ray system is an imaging robot with 5-axis supporting 2D and 3D imaging of anatomical structures and high contrast objects. The navigation feature with both freehand navigation and robotic guidance is based on the standard and established technique of navigation systems using 3D optical position tracking technology, including a stereoscopic camera, the computer platform with monitors, navigation software, a robotic arm and instruments equipped with marker spheres to enable an exact localization in space.
Source: eCential Robotics
eCential Robotics was granted FDA 510(k) clearance for its 3D imaging, navigation and robotics guidance system.
eCential Robotics previously obtained CE Mark approval and launched its first platform in France. Ten units have been sold and installed in Europe, with more than two thousand surgeries performed. FDA 510(k) clearance of the...
eCential Robotics was granted FDA 510(k) clearance for its 3D imaging, navigation and robotics guidance system.
eCential Robotics previously obtained CE Mark approval and launched its first platform in France. Ten units have been sold and installed in Europe, with more than two thousand surgeries performed. FDA 510(k) clearance of the intra-operative 2D and 3D imaging, navigation and robotic guidance platform, with a first universal application in spine surgery, now secures the plan to access the North American market over the next few years.
The platform unifies intraoperative 2D/3D imaging, navigation and robotics and avoids the pitfalls of traditional image-navigation pairing, such as unreliable calibration and registration steps, and streamlines the surgical workflow by automating numerous technical steps. A single user interface for all devices enables to control imaging, navigation and robotic functionalities from a single input and output graphical display screen.
The system is also a fully open solution, meaning that it can be used with any manufacturer’s implants. Built around a range of applications currently dedicated to spine surgery, the eCential Robotics platform will in the future expand to multiple bone surgery indications.
The unified open platform provides 2D/3D medical imaging and stereotaxic guidance. The system consists of three mobile interconnected units: a mobile C-arm, a mobile viewing workstation and a mobile collaborative robot. The mobile X-ray system is an imaging robot with 5-axis supporting 2D and 3D imaging of anatomical structures and high contrast objects. The navigation feature with both freehand navigation and robotic guidance is based on the standard and established technique of navigation systems using 3D optical position tracking technology, including a stereoscopic camera, the computer platform with monitors, navigation software, a robotic arm and instruments equipped with marker spheres to enable an exact localization in space.
Source: eCential Robotics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







