
 Copy to clipboard
Copy to clipboard 
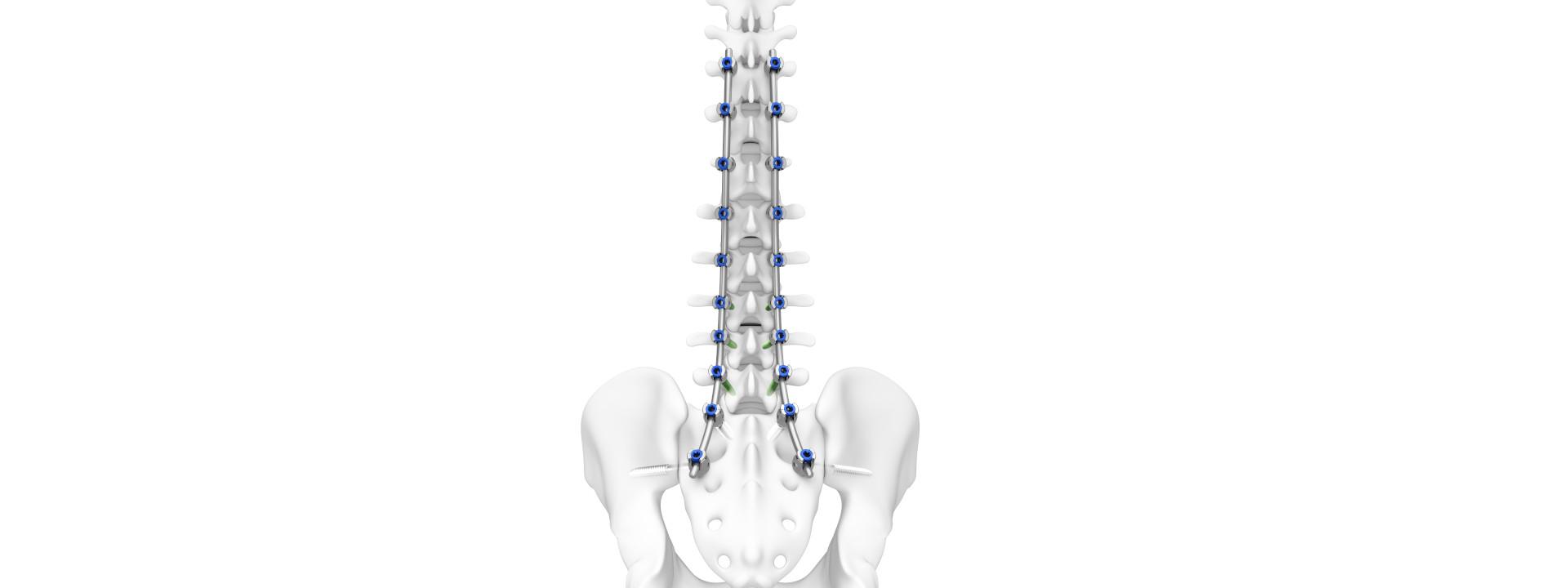
DePuy Synthes secured 510(k) clearances from the FDA for the TriALTIS Spine System and for TriALTIS Navigation Enabled Instruments.
- The TriALTIS Spine System is a next-generation posterior thoracolumbar pedicle screw system that offers a comprehensive implant portfolio and advanced instrumentation designed for integration with enabling technology.
- TriALTIS Navigation Enabled Instruments include drills, taps and screwdrivers that can be operated manually or under power for navigated and non-navigated use.
Combining a new portfolio of implants with a digital ecosystem, the TriALTIS Spine System aims to address unmet clinical needs and help surgeons achieve more consistent outcomes in treating complex spine conditions, inclusive of degenerative, tumor, trauma and deformity pathologies.
Built on a legacy of reliable thoracolumbar solutions and expertise, the TriALTIS Spine System was developed with a hyper-focus on performance and a consistent user experience. The inclusive and forward-looking design philosophy of the TriALTIS Spine System affords smooth integration with cement augmentation, power, navigation and robotic-assisted solutions.
“The TriALTIS Spine System will be our premier pedicle screw platform designed to work seamlessly across our entire DePuy Synthes portfolio of enabling technology solutions,” said Russell Powers, Worldwide President, Spine, DePuy Synthes. “Johnson & Johnson MedTech is proud to deliver this exciting innovation that can help facilitate the surgical treatment of spinal degeneration and deformity with the goal to improve fixation, drive better patient outcomes and potentially reduce the clinical and economic burden associated with the current standard of care. DePuy Synthes has been a leader in the complex spine space for over 35 years, and the launch of the TriALTIS Spine System will mark a critical milestone towards our vision to continue this legacy long into the future.”
Source: Johnson & Johnson
DePuy Synthes secured 510(k) clearances from the FDA for the TriALTIS Spine System and for TriALTIS Navigation Enabled Instruments.
The TriALTIS Spine System is a next-generation posterior thoracolumbar pedicle screw system that offers a comprehensive implant portfolio and advanced instrumentation designed for integration with enabling...
DePuy Synthes secured 510(k) clearances from the FDA for the TriALTIS Spine System and for TriALTIS Navigation Enabled Instruments.
- The TriALTIS Spine System is a next-generation posterior thoracolumbar pedicle screw system that offers a comprehensive implant portfolio and advanced instrumentation designed for integration with enabling technology.
- TriALTIS Navigation Enabled Instruments include drills, taps and screwdrivers that can be operated manually or under power for navigated and non-navigated use.
Combining a new portfolio of implants with a digital ecosystem, the TriALTIS Spine System aims to address unmet clinical needs and help surgeons achieve more consistent outcomes in treating complex spine conditions, inclusive of degenerative, tumor, trauma and deformity pathologies.
Built on a legacy of reliable thoracolumbar solutions and expertise, the TriALTIS Spine System was developed with a hyper-focus on performance and a consistent user experience. The inclusive and forward-looking design philosophy of the TriALTIS Spine System affords smooth integration with cement augmentation, power, navigation and robotic-assisted solutions.
“The TriALTIS Spine System will be our premier pedicle screw platform designed to work seamlessly across our entire DePuy Synthes portfolio of enabling technology solutions,” said Russell Powers, Worldwide President, Spine, DePuy Synthes. “Johnson & Johnson MedTech is proud to deliver this exciting innovation that can help facilitate the surgical treatment of spinal degeneration and deformity with the goal to improve fixation, drive better patient outcomes and potentially reduce the clinical and economic burden associated with the current standard of care. DePuy Synthes has been a leader in the complex spine space for over 35 years, and the launch of the TriALTIS Spine System will mark a critical milestone towards our vision to continue this legacy long into the future.”
Source: Johnson & Johnson

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
ME
Mike Evers is a Senior Market Analyst and writer with over 15 years of experience in the medical industry, spanning cardiac rhythm management, ER coding and billing, and orthopedics. He joined ORTHOWORLD in 2018, where he provides market analysis and editorial coverage.







