
 Copy to clipboard
Copy to clipboard 
DePuy Synthes commenced U.S. launch of TFNA Augmentation, which the company claims to be the first polymethylmethacrylate (PMMA) cement system with specific trauma device indications, offered exclusively for use with the TFN-ADVANCED® Proximal Femoral Nail (TFNA) to address poor bone quality.
When used with the TFNA nail, TFNA Augmentation is intended to reduce the risk of cut-out—a loss of implant stability in the bone—and provide enhanced implant fixation.
DePuy’s TFNA nail debuted in 1Q15, and has since remained a consistently-cited supporter of the company’s trauma sales.
Sources: DePuy Synthes; ORTHOWORLD Inc.
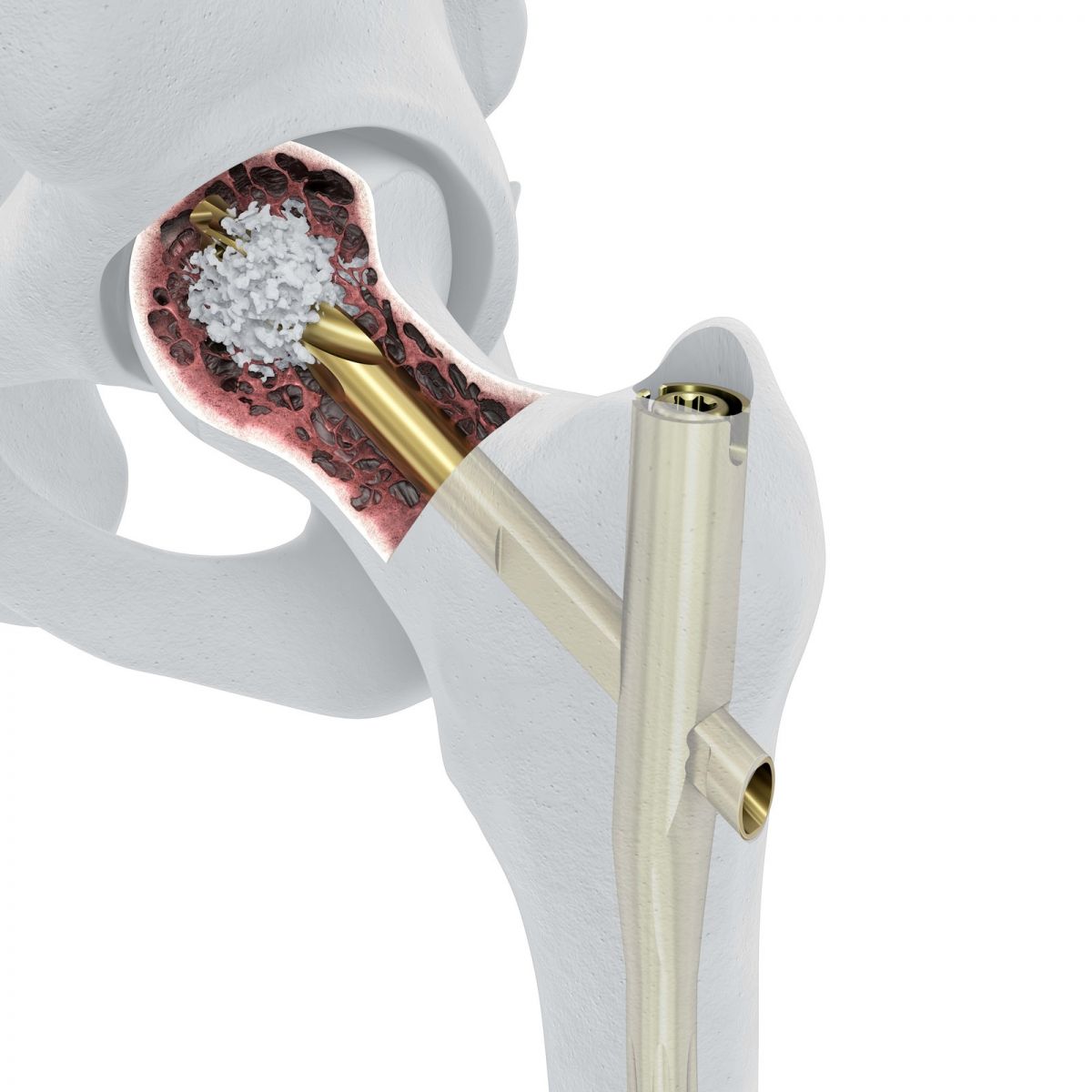
Image courtesy of DePuy Synthes
DePuy Synthes commenced U.S. launch of TFNA Augmentation, which the company claims to be the first polymethylmethacrylate (PMMA) cement system with specific trauma device indications, offered exclusively for use with the TFN-ADVANCED® Proximal Femoral Nail (TFNA) to address poor bone quality.
When used with the TFNA nail, TFNA Augmentation is...
DePuy Synthes commenced U.S. launch of TFNA Augmentation, which the company claims to be the first polymethylmethacrylate (PMMA) cement system with specific trauma device indications, offered exclusively for use with the TFN-ADVANCED® Proximal Femoral Nail (TFNA) to address poor bone quality.
When used with the TFNA nail, TFNA Augmentation is intended to reduce the risk of cut-out—a loss of implant stability in the bone—and provide enhanced implant fixation.
DePuy’s TFNA nail debuted in 1Q15, and has since remained a consistently-cited supporter of the company’s trauma sales.
Sources: DePuy Synthes; ORTHOWORLD Inc.

Image courtesy of DePuy Synthes

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







