
 Copy to clipboard
Copy to clipboard 
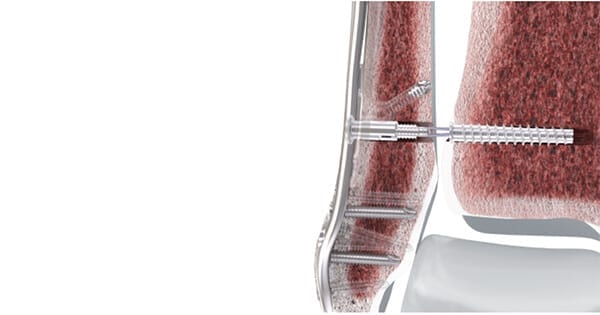
DePuy Synthes launched the FIBULINK® Syndesmosis Repair System in the U.S. to treat traumatic injuries to the syndesmosis. DePuy Synthes acquired the technology earlier this year from Akros Medical.
FIBULINK is the first system to offer a short high-strength suture bridge, which eliminates the risk of complications associated with broken syndesmotic screws. It offers the ability to fine tune and readjust tension intraoperatively, allowing a surgeon to tighten and reverse the tension on the suture as needed to optimize the final gap between the tibia and fibula.
FIBULINK implants are offered in stainless steel or titanium, provided in sterile, single-use kits. It is compatible with all DePuy Synthes distal fibula plates and any plate hole that accepts a 4 mm non-locking cortex screw.
“The acquisition of Akros and subsequent launch of this key technology demonstrate a clear focus on accelerating meaningful innovation and strengthening DePuy Synthes’ Extremities portfolio – providing diverse solutions that enable customers to provide greater benefit to their patients,” said I.V. Hall, Worldwide President, Trauma, Extremities, CMF and Animal Health, DePuy Synthes. “This launch allows us to bring a differentiated solution that combines the benefits of stability and flexibility in the treatment of these common injuries.”
DePuy Synthes launched the FIBULINK® Syndesmosis Repair System in the U.S. to treat traumatic injuries to the syndesmosis. DePuy Synthes acquired the technology earlier this year from Akros Medical.
FIBULINK is the first system to offer a short high-strength suture bridge, which eliminates the risk of complications associated with broken...
DePuy Synthes launched the FIBULINK® Syndesmosis Repair System in the U.S. to treat traumatic injuries to the syndesmosis. DePuy Synthes acquired the technology earlier this year from Akros Medical.
FIBULINK is the first system to offer a short high-strength suture bridge, which eliminates the risk of complications associated with broken syndesmotic screws. It offers the ability to fine tune and readjust tension intraoperatively, allowing a surgeon to tighten and reverse the tension on the suture as needed to optimize the final gap between the tibia and fibula.
FIBULINK implants are offered in stainless steel or titanium, provided in sterile, single-use kits. It is compatible with all DePuy Synthes distal fibula plates and any plate hole that accepts a 4 mm non-locking cortex screw.
“The acquisition of Akros and subsequent launch of this key technology demonstrate a clear focus on accelerating meaningful innovation and strengthening DePuy Synthes’ Extremities portfolio – providing diverse solutions that enable customers to provide greater benefit to their patients,” said I.V. Hall, Worldwide President, Trauma, Extremities, CMF and Animal Health, DePuy Synthes. “This launch allows us to bring a differentiated solution that combines the benefits of stability and flexibility in the treatment of these common injuries.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







