
 Copy to clipboard
Copy to clipboard 
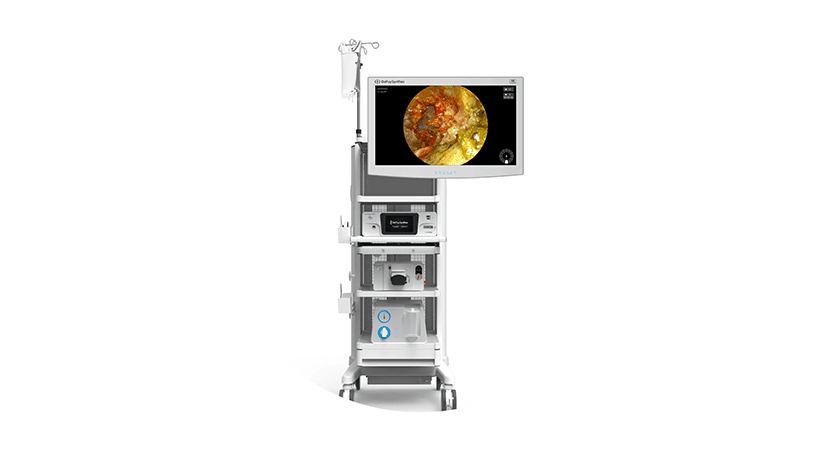
DePuy Synthes was granted FDA 510(k) clearance to market the TELIGEN™ System, an integrated technology platform that supports minimally invasive surgical transforaminal lumbar interbody fusion (MIS-TLIF) procedures through digital tools for visualization and access.
TELIGEN will be available in the U.S. later this year.
TELIGEN comprises a tower that delivers a suite of technologies, including camera control, a VueLIF-T™ Procedure Kit with disposable HD camera, a TELIGEN Clear Discectomy Device and patient-based disposable ports. TELIGEN integrates with the UNLEASH™ bundle of implants, which is designed to streamline the main stages in MIS-TLIF.
The digitally enabled TELIGEN VUE Camera, located at the distal end of the patient-specific port, is designed to eliminate the need for a microscope and can provide an unobstructed view of the surgical site. It offers hands-free visualization during the procedure, along with a multidirectional and expanded field of view. Additionally, the self-cleaning camera includes LED lighting and allows surgeons to manipulate image clarity based on their preference. A heads-up display allows surgeons to maintain ergonomic posture during procedures.
Based on a small cadaveric study, TELIGEN can reduce fluoroscopy time by 47% compared to MIS-TLIF procedures performed using a surgical microscope. It also provides a reduction in instrument trays and processing costs per surgery for the 10 additional trays not required with the TELIGEN™ System.
Source: DePuy Synthes
DePuy Synthes was granted FDA 510(k) clearance to market the TELIGEN™ System, an integrated technology platform that supports minimally invasive surgical transforaminal lumbar interbody fusion (MIS-TLIF) procedures through digital tools for visualization and access.
TELIGEN will be available in the U.S. later this year.
TELIGEN comprises a...
DePuy Synthes was granted FDA 510(k) clearance to market the TELIGEN™ System, an integrated technology platform that supports minimally invasive surgical transforaminal lumbar interbody fusion (MIS-TLIF) procedures through digital tools for visualization and access.
TELIGEN will be available in the U.S. later this year.
TELIGEN comprises a tower that delivers a suite of technologies, including camera control, a VueLIF-T™ Procedure Kit with disposable HD camera, a TELIGEN Clear Discectomy Device and patient-based disposable ports. TELIGEN integrates with the UNLEASH™ bundle of implants, which is designed to streamline the main stages in MIS-TLIF.
The digitally enabled TELIGEN VUE Camera, located at the distal end of the patient-specific port, is designed to eliminate the need for a microscope and can provide an unobstructed view of the surgical site. It offers hands-free visualization during the procedure, along with a multidirectional and expanded field of view. Additionally, the self-cleaning camera includes LED lighting and allows surgeons to manipulate image clarity based on their preference. A heads-up display allows surgeons to maintain ergonomic posture during procedures.
Based on a small cadaveric study, TELIGEN can reduce fluoroscopy time by 47% compared to MIS-TLIF procedures performed using a surgical microscope. It also provides a reduction in instrument trays and processing costs per surgery for the 10 additional trays not required with the TELIGEN™ System.
Source: DePuy Synthes

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







