
 Copy to clipboard
Copy to clipboard 
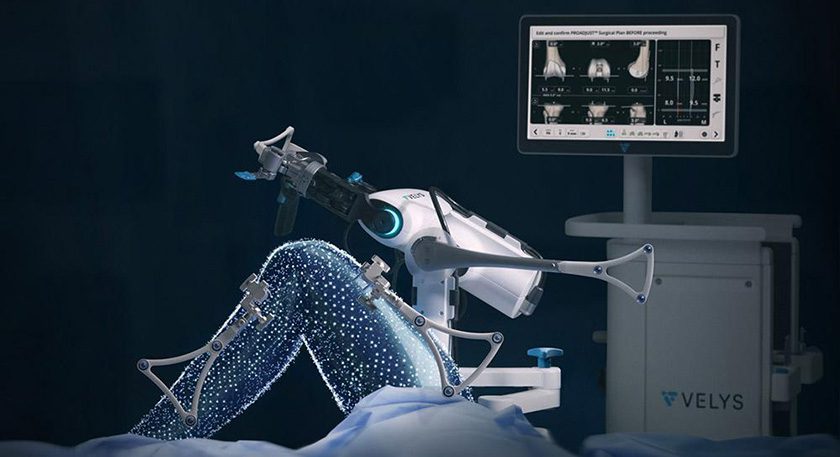
DePuy Synthes received FDA 510(k) clearance for the clinical application of the VELYS Robotic-Assisted Solution in unicompartmental knee arthroplasty (UKA). The expanded indication builds upon the VELYS Robotic-Assisted Solution platform used in total knee arthroplasty (TKA), which has been cleared for use in 20 markets and utilized in over 55,000 procedures.
The UKA application is designed for both medial and lateral procedures and will enable surgeons to guide precise implant placement without a CT scan. It is compatible with the SIGMA HP Unicondylar Knee with the new reusable INTUITION INSTRUMENTS.
Key features of the VELYS Robotic-Assisted Solution for UKA include:
- The single PROADJUST PLANNING SCREEN enables personalized planning to help ensure precise implant placement, alignment and balance relative to soft tissue.
- An ACCUBALANCE GRAPH for personalized balancing throughout the full range of motion.
- Complete UKA workflow with UKA-specific functionality.
“We are committed to continually improving and expanding the capabilities and user experience of our VELYS Enabling Technology portfolio,” said Aldo Denti, Company Group Chairman, DePuy Synthes. “We are excited to add a robotic-assisted offering with our clinically proven implant for UKA which we believe will address some of the key unmet needs in the partial knee replacement segment, including accuracy and simplicity, that other systems on the market do not fully address. Data and analytics will continue to serve as the backbone of our platform, which reveals real-time, actionable insights for surgeons to empower patient-specific operative decisions with the goal to improve outcomes and deliver personalization at scale.”
Source: DePuy Synthes
DePuy Synthes received FDA 510(k) clearance for the clinical application of the VELYS Robotic-Assisted Solution in unicompartmental knee arthroplasty (UKA). The expanded indication builds upon the VELYS Robotic-Assisted Solution platform used in total knee arthroplasty (TKA), which has been cleared for use in 20 markets and utilized in over...
DePuy Synthes received FDA 510(k) clearance for the clinical application of the VELYS Robotic-Assisted Solution in unicompartmental knee arthroplasty (UKA). The expanded indication builds upon the VELYS Robotic-Assisted Solution platform used in total knee arthroplasty (TKA), which has been cleared for use in 20 markets and utilized in over 55,000 procedures.
The UKA application is designed for both medial and lateral procedures and will enable surgeons to guide precise implant placement without a CT scan. It is compatible with the SIGMA HP Unicondylar Knee with the new reusable INTUITION INSTRUMENTS.
Key features of the VELYS Robotic-Assisted Solution for UKA include:
- The single PROADJUST PLANNING SCREEN enables personalized planning to help ensure precise implant placement, alignment and balance relative to soft tissue.
- An ACCUBALANCE GRAPH for personalized balancing throughout the full range of motion.
- Complete UKA workflow with UKA-specific functionality.
“We are committed to continually improving and expanding the capabilities and user experience of our VELYS Enabling Technology portfolio,” said Aldo Denti, Company Group Chairman, DePuy Synthes. “We are excited to add a robotic-assisted offering with our clinically proven implant for UKA which we believe will address some of the key unmet needs in the partial knee replacement segment, including accuracy and simplicity, that other systems on the market do not fully address. Data and analytics will continue to serve as the backbone of our platform, which reveals real-time, actionable insights for surgeons to empower patient-specific operative decisions with the goal to improve outcomes and deliver personalization at scale.”
Source: DePuy Synthes

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







