
 Copy to clipboard
Copy to clipboard 
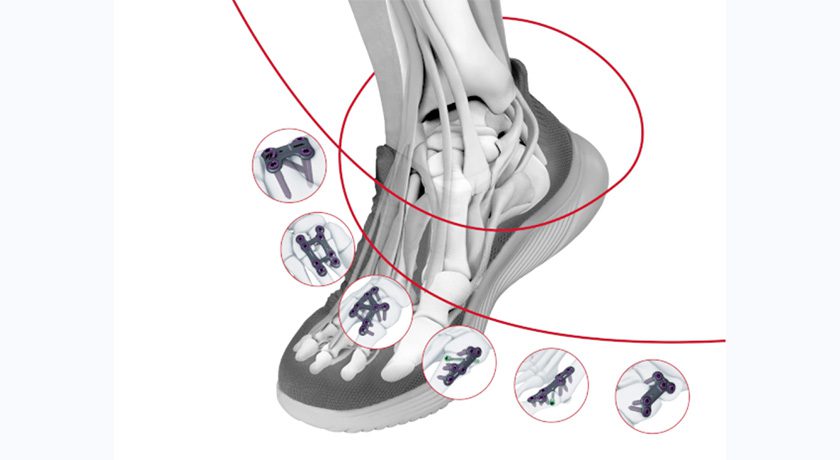
DePuy Synthes received FDA 510(k) clearance to market the TriLEAP Lower Extremity Anatomic Plating System, a modular, procedure-specific system of contoured and conventional plates that can accommodate multiple screw diameters, and instruments that can be used during the reduction, internal fixation and fusion of bones and bone fragments.
TriLEAP offers multiple procedure-specific plate options to cover a wide range of procedures, as well as an extensive procedure‐specific implant choice with multiple screw shaft diameters that provide surgeons with various options for intraoperative decisions.
U.S. system launch is slated to occur during 2024.
Source: DePuy Synthes
DePuy Synthes received FDA 510(k) clearance to market the TriLEAP Lower Extremity Anatomic Plating System, a modular, procedure-specific system of contoured and conventional plates that can accommodate multiple screw diameters, and instruments that can be used during the reduction, internal fixation and fusion of bones and bone fragments.
...
DePuy Synthes received FDA 510(k) clearance to market the TriLEAP Lower Extremity Anatomic Plating System, a modular, procedure-specific system of contoured and conventional plates that can accommodate multiple screw diameters, and instruments that can be used during the reduction, internal fixation and fusion of bones and bone fragments.
TriLEAP offers multiple procedure-specific plate options to cover a wide range of procedures, as well as an extensive procedure‐specific implant choice with multiple screw shaft diameters that provide surgeons with various options for intraoperative decisions.
U.S. system launch is slated to occur during 2024.
Source: DePuy Synthes

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







