
 Copy to clipboard
Copy to clipboard 
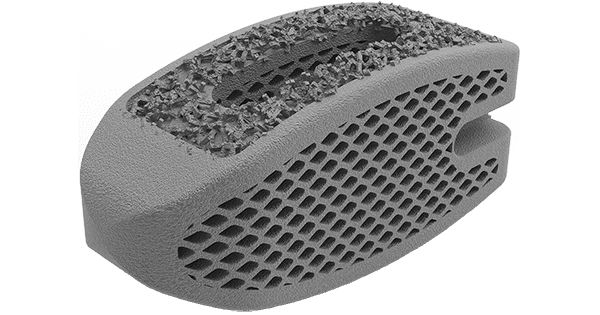
DeGen Medical launched Impulse AM™, a 3D-printed porous titanium implant for posterior spinal interbody fusion. Impulse AM is reportedly the first 3D-printed spinal implant to employ Puri-Ti™, a different type of titanium material with a proprietary manufacturing process.
This product release provides implants and instrumentation designed for PLIF and TLIF fusion procedures. Impulse AM comes in a variety of sizes and anatomical designs to accommodate patient anatomy.
Highlights of the Puri-Ti Technology Include:
Proprietary “Green” Manufacturing Process: Throughout the entire manufacturing and post processing steps no cutting oils, fluids or heat treatment is required. This in conjunction with the Puri-Ti proprietary material is a completely “green” process that will shorten lead times for patient specific implants. It took three years to perfect the laser parameters and chemistry to create the Puri-Ti material. DeGen Medical is first company to break the mold and create a game changing process and material in medical device additive manufacturing space.
Patient Specific Implants: DeGen Medical will be able to create patient specific implants at a fraction of the time and cost of current products. Currently available products are utilized as one-offs and not for everyday surgical interventions. DeGen Medical plans to change this and become a market leader in patient specific solutions.
Source: DeGen Medical
DeGen Medical launched Impulse AM™, a 3D-printed porous titanium implant for posterior spinal interbody fusion. Impulse AM is reportedly the first 3D-printed spinal implant to employ Puri-Ti™, a different type of titanium material with a proprietary manufacturing process.
This product release provides implants and instrumentation designed for...
DeGen Medical launched Impulse AM™, a 3D-printed porous titanium implant for posterior spinal interbody fusion. Impulse AM is reportedly the first 3D-printed spinal implant to employ Puri-Ti™, a different type of titanium material with a proprietary manufacturing process.
This product release provides implants and instrumentation designed for PLIF and TLIF fusion procedures. Impulse AM comes in a variety of sizes and anatomical designs to accommodate patient anatomy.
Highlights of the Puri-Ti Technology Include:
Proprietary “Green” Manufacturing Process: Throughout the entire manufacturing and post processing steps no cutting oils, fluids or heat treatment is required. This in conjunction with the Puri-Ti proprietary material is a completely “green” process that will shorten lead times for patient specific implants. It took three years to perfect the laser parameters and chemistry to create the Puri-Ti material. DeGen Medical is first company to break the mold and create a game changing process and material in medical device additive manufacturing space.
Patient Specific Implants: DeGen Medical will be able to create patient specific implants at a fraction of the time and cost of current products. Currently available products are utilized as one-offs and not for everyday surgical interventions. DeGen Medical plans to change this and become a market leader in patient specific solutions.
Source: DeGen Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







