
 Copy to clipboard
Copy to clipboard 
DeGen Medical received FDA clearance to market the F1 MP’s Modular Pedicle Screw System for JOUST™ Minimally Invasive Surgery and the Cyclops’ Anterior Cervical Plate.
JOUST employs MIS Towers for percutaneous screw placement in the traditional or cortical approach, with built-in rod reduction. JOUST enables screw placement with minimal muscle stripping, leading to the formation of less scar tissue.
The JOUST™ MIS technology with F1 MP’s System will be available in Q4 of 2019.
Cyclops is a short, low profile plate available in multiple lengths, with hyper-angulated screws to support fixed, variable or hybrid constructs.
Both JOUST and Cyclops will launch in 4Q19.
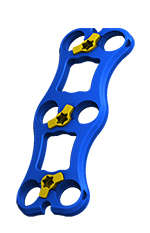 |
| Cyclops Anterior Cervical Plate |
DeGen Medical received FDA clearance to market the F1 MP's Modular Pedicle Screw System for JOUST™ Minimally Invasive Surgery and the Cyclops' Anterior Cervical Plate.
JOUST employs MIS Towers for percutaneous screw placement in the traditional or cortical approach, with built-in rod reduction. JOUST enables screw placement with minimal muscle...
DeGen Medical received FDA clearance to market the F1 MP’s Modular Pedicle Screw System for JOUST™ Minimally Invasive Surgery and the Cyclops’ Anterior Cervical Plate.
JOUST employs MIS Towers for percutaneous screw placement in the traditional or cortical approach, with built-in rod reduction. JOUST enables screw placement with minimal muscle stripping, leading to the formation of less scar tissue.
The JOUST™ MIS technology with F1 MP’s System will be available in Q4 of 2019.
Cyclops is a short, low profile plate available in multiple lengths, with hyper-angulated screws to support fixed, variable or hybrid constructs.
Both JOUST and Cyclops will launch in 4Q19.
 |
| Cyclops Anterior Cervical Plate |
Source: DeGen Medical Cyclops; DeGen Medical F1 MPS

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







