
 Copy to clipboard
Copy to clipboard 
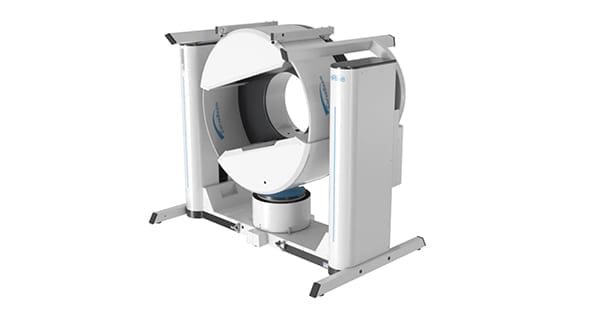
CurveBeam received FDA 510(k) clearance to market the HiRise™ weight bearing CT (WBCT) imaging system for the entire lower extremity.
HiRise can scan anywhere along the lower extremity from the hip/pelvis to the feet. WBCT imaging of the hip and pelvis is a novel application not previously available in any system. The HiRise gantry tilts for upper extremity imaging and supine lower leg imaging.
The system is employed in multiple ongoing research studies, including examination of hip dysplasia in functional position and evaluating wrist injuries in gymnasts.
CurveBeam systems utilize cone beam CT technology and are designed for the orthopedic point-of-care setting. HiRise plugs into a standard wall outlet, requires minimal shielding and is easy to operate.
CurveBeam received CE Mark approval for the HiRise earlier this year.
“CurveBeam’s mission is to be responsive to crucial but unmet diagnostic needs of the specialties it serves, and introduction of the HiRise device is the latest transformational step in that direction,” said CurveBeam President & CEO Arun Singh. “This system literally achieves new heights in point of care advanced orthopedic imaging.”
CurveBeam received FDA 510(k) clearance to market the HiRise™ weight bearing CT (WBCT) imaging system for the entire lower extremity.
HiRise can scan anywhere along the lower extremity from the hip/pelvis to the feet. WBCT imaging of the hip and pelvis is a novel application not previously available in any system. The HiRise gantry tilts...
CurveBeam received FDA 510(k) clearance to market the HiRise™ weight bearing CT (WBCT) imaging system for the entire lower extremity.
HiRise can scan anywhere along the lower extremity from the hip/pelvis to the feet. WBCT imaging of the hip and pelvis is a novel application not previously available in any system. The HiRise gantry tilts for upper extremity imaging and supine lower leg imaging.
The system is employed in multiple ongoing research studies, including examination of hip dysplasia in functional position and evaluating wrist injuries in gymnasts.
CurveBeam systems utilize cone beam CT technology and are designed for the orthopedic point-of-care setting. HiRise plugs into a standard wall outlet, requires minimal shielding and is easy to operate.
CurveBeam received CE Mark approval for the HiRise earlier this year.
“CurveBeam’s mission is to be responsive to crucial but unmet diagnostic needs of the specialties it serves, and introduction of the HiRise device is the latest transformational step in that direction,” said CurveBeam President & CEO Arun Singh. “This system literally achieves new heights in point of care advanced orthopedic imaging.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







