Copy to clipboard
Copy to clipboard 
CurvaFix received FDA 510(k) clearance to market the CurvaFix® Intramedullary Rodscrew for pelvic trauma surgery.
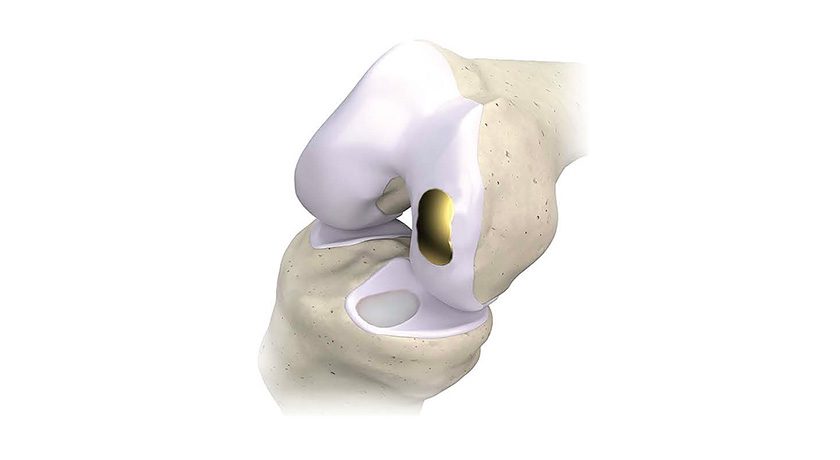
The flexible device is implanted through a small incision into the intramedullary space, then converted into a rigid state to stabilize and repair the fracture. The CurvaFix Rodscrew is reportedly the only intramedullary implant capable of following the natural bone shape of curved bones, such as the pelvis.
“We believe surgeons will welcome an implantable device that adapts to the patient’s own bone curvature and we anticipate that the CurvaFix Rodscrew will improve fixation, shorten surgeries and reduce care costs,” said Steve Dimmer, CEO for CurvaFix.
Founded in 2013, CurvaFix has raised a total of $2.5M in funding over two rounds to date, with the latest occuring in 2018.
Source: CurvaFix, Inc.
CurvaFix received FDA 510(k) clearance to market the CurvaFix® Intramedullary Rodscrew for pelvic trauma surgery.
The flexible device is implanted through a small incision into the intramedullary space, then converted into a rigid state to stabilize and repair the fracture. The CurvaFix Rodscrew is reportedly the only intramedullary implant...
CurvaFix received FDA 510(k) clearance to market the CurvaFix® Intramedullary Rodscrew for pelvic trauma surgery.
The flexible device is implanted through a small incision into the intramedullary space, then converted into a rigid state to stabilize and repair the fracture. The CurvaFix Rodscrew is reportedly the only intramedullary implant capable of following the natural bone shape of curved bones, such as the pelvis.
“We believe surgeons will welcome an implantable device that adapts to the patient’s own bone curvature and we anticipate that the CurvaFix Rodscrew will improve fixation, shorten surgeries and reduce care costs,” said Steve Dimmer, CEO for CurvaFix.
Founded in 2013, CurvaFix has raised a total of $2.5M in funding over two rounds to date, with the latest occuring in 2018.
Source: CurvaFix, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.