
 Copy to clipboard
Copy to clipboard 
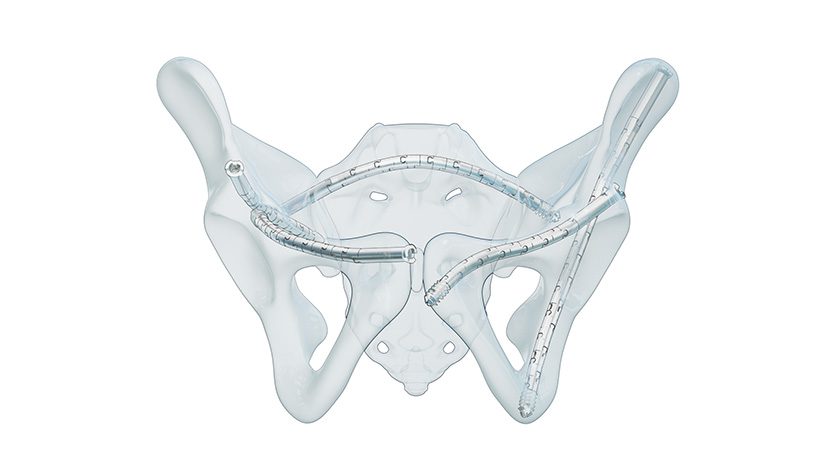
CurvaFix was granted FDA 510(k) clearance for its next-generation CurvaFix Low Profile System, a percutaneous solution for fixation of pelvic fractures.
The CurvaFix Low Profile System is built to expand surgical options in challenging scenarios, including pathological bone, curved or narrow pelvic corridors, intersecting fixation pathways and cases where indwelling or adjacent hardware is present. The new system offers:
- 65% smaller head geometry for a lower-profile construct
- Implants with increased compression capability
- Extended implant lengths up to 210mm, enabling connection of intraosseous fixation pathways with a single device
- Patented lock technology with improved visual and tactile confirmation
Clinical experience has shown that the CurvaFix technology provides strong, stable fixation that follows and fills the natural curvature of each patient’s anatomy. This unique approach may reduce pain, support earlier mobility and improve recovery compared to conventional straight implants.
“CurvaFix continues to revolutionize pelvic fracture fixation with the launch of our Low-Profile System,” said Mark Foster, CEO of CurvaFix. “This new system reflects four years of clinical use and thousands of successful implants, coupled with direct feedback from orthopedic trauma surgeons. The result is a familiar, intuitive implantation technique designed to address the complexities of pelvic fracture fixation, while also allowing streamlined implant removal if required.”
Source: CurvaFix, Inc.
CurvaFix was granted FDA 510(k) clearance for its next-generation CurvaFix Low Profile System, a percutaneous solution for fixation of pelvic fractures.
The CurvaFix Low Profile System is built to expand surgical options in challenging scenarios, including pathological bone, curved or narrow pelvic corridors, intersecting fixation pathways and...
CurvaFix was granted FDA 510(k) clearance for its next-generation CurvaFix Low Profile System, a percutaneous solution for fixation of pelvic fractures.
The CurvaFix Low Profile System is built to expand surgical options in challenging scenarios, including pathological bone, curved or narrow pelvic corridors, intersecting fixation pathways and cases where indwelling or adjacent hardware is present. The new system offers:
- 65% smaller head geometry for a lower-profile construct
- Implants with increased compression capability
- Extended implant lengths up to 210mm, enabling connection of intraosseous fixation pathways with a single device
- Patented lock technology with improved visual and tactile confirmation
Clinical experience has shown that the CurvaFix technology provides strong, stable fixation that follows and fills the natural curvature of each patient’s anatomy. This unique approach may reduce pain, support earlier mobility and improve recovery compared to conventional straight implants.
“CurvaFix continues to revolutionize pelvic fracture fixation with the launch of our Low-Profile System,” said Mark Foster, CEO of CurvaFix. “This new system reflects four years of clinical use and thousands of successful implants, coupled with direct feedback from orthopedic trauma surgeons. The result is a familiar, intuitive implantation technique designed to address the complexities of pelvic fracture fixation, while also allowing streamlined implant removal if required.”
Source: CurvaFix, Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







