
 Copy to clipboard
Copy to clipboard 
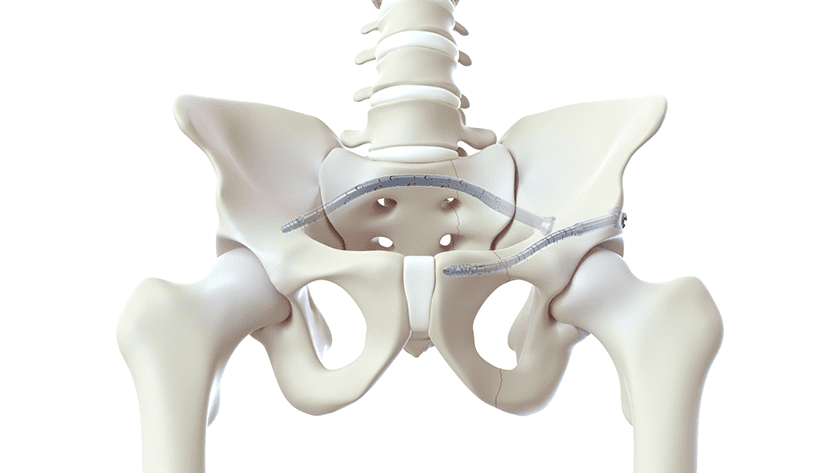
CurvaFix introduced its smaller-diameter, 7.5mm CurvaFix IM Implant, designed to simplify surgery and provide strong, stable fixation in small-boned patients. The device received FDA market clearance in late 2022.
To date over 175 patients have been treated with the CurvaFix procedure, including over 100 patients who are either geriatric and/or suffer from Fragility Fractures of the Pelvis (FFP). Usage of over 240 CurvaFix Implants, by 35 U.S. surgeons, suggests the advantages of a longer, wider, curved implant, which may immediately reduce pain and enable early mobility in a broad range of patients through a minimally invasive procedure.
The patient population demonstrates surgical utility and potential benefits in a variety of pelvic injuries and conditions, including polytrauma patients with multiple complex injuries, FFP patients with weak bone, patients with dysmorphic bony anatomy, oncology patients with pelvic fractures, revision surgery for failed pelvic fracture fixation, and patients with impeding total hip or lumbosacral spinal hardware.
“The new 7.5mm device is designed to simplify surgery and provide strong, stable, curved fixation in smaller patients,” said Steve Dimmer, Chief Executive Officer for CurvaFix. “Additionally, our novel device has been shown to offer many geriatric patients immediate pain relief and early mobility, which is critically important in older patients where mobility is such a key to life.”
Source: CurvaFix, Inc.
CurvaFix introduced its smaller-diameter, 7.5mm CurvaFix IM Implant, designed to simplify surgery and provide strong, stable fixation in small-boned patients. The device received FDA market clearance in late 2022.
To date over 175 patients have been treated with the CurvaFix procedure, including over 100 patients who are either geriatric...
CurvaFix introduced its smaller-diameter, 7.5mm CurvaFix IM Implant, designed to simplify surgery and provide strong, stable fixation in small-boned patients. The device received FDA market clearance in late 2022.
To date over 175 patients have been treated with the CurvaFix procedure, including over 100 patients who are either geriatric and/or suffer from Fragility Fractures of the Pelvis (FFP). Usage of over 240 CurvaFix Implants, by 35 U.S. surgeons, suggests the advantages of a longer, wider, curved implant, which may immediately reduce pain and enable early mobility in a broad range of patients through a minimally invasive procedure.
The patient population demonstrates surgical utility and potential benefits in a variety of pelvic injuries and conditions, including polytrauma patients with multiple complex injuries, FFP patients with weak bone, patients with dysmorphic bony anatomy, oncology patients with pelvic fractures, revision surgery for failed pelvic fracture fixation, and patients with impeding total hip or lumbosacral spinal hardware.
“The new 7.5mm device is designed to simplify surgery and provide strong, stable, curved fixation in smaller patients,” said Steve Dimmer, Chief Executive Officer for CurvaFix. “Additionally, our novel device has been shown to offer many geriatric patients immediate pain relief and early mobility, which is critically important in older patients where mobility is such a key to life.”
Source: CurvaFix, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







