
 Copy to clipboard
Copy to clipboard 
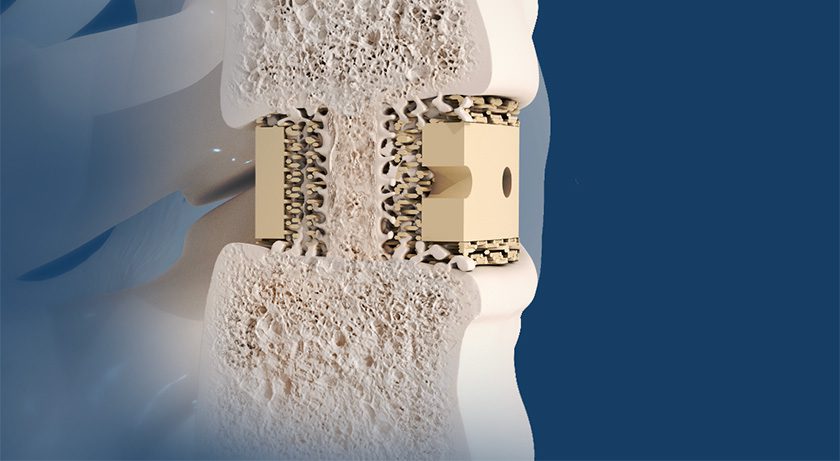
Curiteva has implanted over 2,000 patients with the Inspire C Cervical Interbody Fusion Device. This translates to more than 3,700 implants placed, demonstrating the system’s rapid adoption since its commercial launch in July 2023.
The Inspire platform is manufactured with a proprietary, patented Fused Strand Deposition 3D printer designed, programmed and built by Curiteva. The first-to-market combination of the HAFUSE sub-micron surface texture and novel porous PEEK structure presents a hydrophilic, bioactive environment for cell attachment, proliferation and healing in preclinical animal and in vitro studies.
“The most exciting thing about reaching this 2,000-patient milestone is realizing the outstanding preliminary outcomes as our ongoing collection of data continues to validate and underscore the novelty of this Inspire Technology,” commented Curiteva’s Chief Scientific Officer, Erik Erbe, PhD. “Most recently the data was shared in ‘Unique Osseointegration Patterns in 3D Printed Trabecular-Architecture Porous Polyetheretherketone (PEEK). A CT Scan Study of the Inspire Cervical Implant’.”
Source: Curiteva
Curiteva has implanted over 2,000 patients with the Inspire C Cervical Interbody Fusion Device. This translates to more than 3,700 implants placed, demonstrating the system's rapid adoption since its commercial launch in July 2023.
The Inspire platform is manufactured with a proprietary, patented Fused Strand Deposition 3D printer designed,...
Curiteva has implanted over 2,000 patients with the Inspire C Cervical Interbody Fusion Device. This translates to more than 3,700 implants placed, demonstrating the system’s rapid adoption since its commercial launch in July 2023.
The Inspire platform is manufactured with a proprietary, patented Fused Strand Deposition 3D printer designed, programmed and built by Curiteva. The first-to-market combination of the HAFUSE sub-micron surface texture and novel porous PEEK structure presents a hydrophilic, bioactive environment for cell attachment, proliferation and healing in preclinical animal and in vitro studies.
“The most exciting thing about reaching this 2,000-patient milestone is realizing the outstanding preliminary outcomes as our ongoing collection of data continues to validate and underscore the novelty of this Inspire Technology,” commented Curiteva’s Chief Scientific Officer, Erik Erbe, PhD. “Most recently the data was shared in ‘Unique Osseointegration Patterns in 3D Printed Trabecular-Architecture Porous Polyetheretherketone (PEEK). A CT Scan Study of the Inspire Cervical Implant’.”
Source: Curiteva

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







