
 Copy to clipboard
Copy to clipboard 
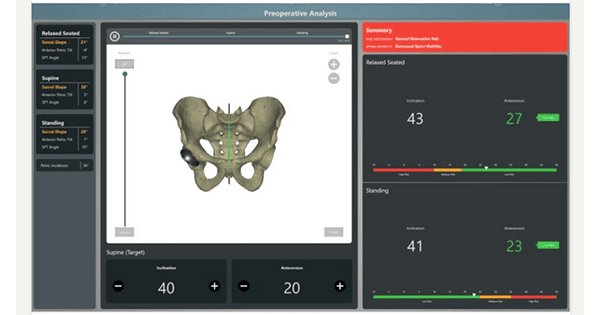
Cuptimize received FDA 510(k) clearance for spinopelvic tilt analysis software that provides a preoperative and intraoperative solution for hip replacement. The software is now available in the U.S.
The software relies exclusively on x-ray, and requires no CT scan for the preoperative analysis module.
Cuptimize allows hip surgeons to understand when the patient has a problem, determine cup placement to mitigate risk and have clear indications of when to use dual mobility implants. The system also integrates directly with intraoperative navigation solutions.
“With Cuptimize,” said Dr. Andrew J. Cooper, M.D., “surgeons can preoperatively plan their patient’s specific cup position simply with x-rays. In the operating room, the fully integrated solution allows the cup to be seen, not as a singular position, but as a range from sitting to standing. This should bring confidence to surgeons of all approaches when implanting their acetabular component.”
Cuptimize received FDA 510(k) clearance for spinopelvic tilt analysis software that provides a preoperative and intraoperative solution for hip replacement. The software is now available in the U.S.
The software relies exclusively on x-ray, and requires no CT scan for the preoperative analysis module.
Cuptimize allows hip surgeons to...
Cuptimize received FDA 510(k) clearance for spinopelvic tilt analysis software that provides a preoperative and intraoperative solution for hip replacement. The software is now available in the U.S.
The software relies exclusively on x-ray, and requires no CT scan for the preoperative analysis module.
Cuptimize allows hip surgeons to understand when the patient has a problem, determine cup placement to mitigate risk and have clear indications of when to use dual mobility implants. The system also integrates directly with intraoperative navigation solutions.
“With Cuptimize,” said Dr. Andrew J. Cooper, M.D., “surgeons can preoperatively plan their patient’s specific cup position simply with x-rays. In the operating room, the fully integrated solution allows the cup to be seen, not as a singular position, but as a range from sitting to standing. This should bring confidence to surgeons of all approaches when implanting their acetabular component.”

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







